A new method for quantifying mitochondrial axonal transport
- PMID: 27225265
- PMCID: PMC5084152
- DOI: 10.1007/s13238-016-0268-3
A new method for quantifying mitochondrial axonal transport
Abstract
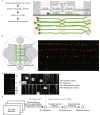
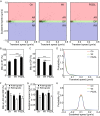
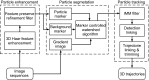
Axonal transport of mitochondria is critical for neuronal survival and function. Automatically quantifying and analyzing mitochondrial movement in a large quantity remain challenging. Here, we report an efficient method for imaging and quantifying axonal mitochondrial transport using microfluidic-chamber-cultured neurons together with a newly developed analysis package named "MitoQuant". This tool-kit consists of an automated program for tracking mitochondrial movement inside live neuronal axons and a transient-velocity analysis program for analyzing dynamic movement patterns of mitochondria. Using this method, we examined axonal mitochondrial movement both in cultured mammalian neurons and in motor neuron axons of Drosophila in vivo. In 3 different paradigms (temperature changes, drug treatment and genetic manipulation) that affect mitochondria, we have shown that this new method is highly efficient and sensitive for detecting changes in mitochondrial movement. The method significantly enhanced our ability to quantitatively analyze axonal mitochondrial movement and allowed us to detect dynamic changes in axonal mitochondrial transport that were not detected by traditional kymographic analyses.
Keywords: FUS proteinopathy and mitochondrial transport defect; image processing and analysis; mitochondrial transport.
Figures



Similar articles
-
Axonal Transport of Organelles in Motor Neuron Cultures using Microfluidic Chambers System.J Vis Exp. 2020 May 5;(159). doi: 10.3791/60993. J Vis Exp. 2020. PMID: 32449725
-
Compartmentalized Regulation of Parkin-Mediated Mitochondrial Quality Control in the Drosophila Nervous System In Vivo.J Neurosci. 2016 Jul 13;36(28):7375-91. doi: 10.1523/JNEUROSCI.0633-16.2016. J Neurosci. 2016. PMID: 27413149 Free PMC article.
-
Developmental changes in trak-mediated mitochondrial transport in neurons.Mol Cell Neurosci. 2017 Apr;80:134-147. doi: 10.1016/j.mcn.2017.03.006. Epub 2017 Mar 11. Mol Cell Neurosci. 2017. PMID: 28300646 Free PMC article.
-
Regulation of axonal mitochondrial transport and its impact on synaptic transmission.Neurosci Res. 2011 May;70(1):9-15. doi: 10.1016/j.neures.2011.02.005. Epub 2011 Feb 23. Neurosci Res. 2011. PMID: 21352858 Free PMC article. Review.
-
The axonal transport of mitochondria.J Cell Sci. 2012 May 1;125(Pt 9):2095-104. doi: 10.1242/jcs.053850. Epub 2012 May 22. J Cell Sci. 2012. PMID: 22619228 Free PMC article. Review.
Cited by
-
Dynamic properties of mitochondria during human corticogenesis.Development. 2021 Feb 19;148(4):dev194183. doi: 10.1242/dev.194183. Development. 2021. PMID: 33608250 Free PMC article. Review.
-
A new microfluidic model to study dendritic remodeling and mitochondrial dynamics during axonal regeneration of adult zebrafish retinal neurons.Front Mol Neurosci. 2023 Jun 15;16:1196504. doi: 10.3389/fnmol.2023.1196504. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37396787 Free PMC article.
-
Analyzing Mitochondrial Transport and Morphology in Human Induced Pluripotent Stem Cell-Derived Neurons in Hereditary Spastic Paraplegia.J Vis Exp. 2020 Feb 9;(156):10.3791/60548. doi: 10.3791/60548. J Vis Exp. 2020. PMID: 32090993 Free PMC article.
-
Syntaphilin Inactivation Can Enhance Axonal Mitochondrial Transport to Improve Spinal Cord Injury.Mol Neurobiol. 2023 Nov;60(11):6556-6565. doi: 10.1007/s12035-023-03494-6. Epub 2023 Jul 17. Mol Neurobiol. 2023. PMID: 37458986 Review.
-
Astrocyte-Derived Interleukin 11 Modulates Astrocyte-Microglia Crosstalk via Nuclear Factor-κB Signaling Pathway in Sepsis-Associated Encephalopathy.Research (Wash D C). 2025 Jan 30;8:0598. doi: 10.34133/research.0598. eCollection 2025. Research (Wash D C). 2025. PMID: 39886603 Free PMC article.
References
-
- Borland MK, Trimmer PA, Rubinstein JD, Keeney PM, Mohanakumar K, Liu L, Bennett JP., Jr Chronic, low-dose rotenone reproduces Lewy neurites found in early stages of Parkinson’s disease, reduces mitochondrial movement and slowly kills differentiated SH-SY5Y neural cells. Mol Neurodegener. 2008;3:21. doi: 10.1186/1750-1326-3-21. - DOI - PMC - PubMed
-
- Buades A, Coll B, Morel JM. A non-local algorithm for image denoising. Comput Vis Pattern Recognit. 2005;2:60–65.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

