Rapamycin-loaded Immunoliposomes Functionalized with Trastuzumab: A Strategy to Enhance Cytotoxicity to HER2-positive Breast Cancer Cells
- PMID: 27225450
- PMCID: PMC5123968
Rapamycin-loaded Immunoliposomes Functionalized with Trastuzumab: A Strategy to Enhance Cytotoxicity to HER2-positive Breast Cancer Cells
Abstract
Background: Liposomes have been employed to improve pharmacokinetics and reduce side effects of drugs. They can be functionalized with antibodies for targeted delivery. While the monoclonal antibody trastuzumab has been employed in the therapy of HER2-positive breast cancer, the resistance developed during treatment has been reported. Rapamycin could be used in combination with trastuzumab for improved therapeutic response.
Objective: In this study, we aimed to develop rapamycin-loaded liposomes and immunoliposomes with trastuzumab, characterize them and evaluate their in vitro cytotoxicity.
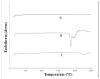
Method: Formulations were prepared by the thin film hydration method and immunoliposome was conjugated to antibody by covalent bond. Characterization involved particle size, polydispersity, zeta potential, encapsulation efficiency, functionalization efficiency, DSC and FTIR assays. Cell studies were conducted through the MTT assay.
Results: SPC:Chol:DSPE-PEG formulation prepared at 1:10 drug to lipid ratio presented high encapsulation efficiency, appropriate particle size, low polydispersity, negative zeta potential and colloidal stability. Rapamycin exhibited intermolecular interactions with lipids and underwent crystallinity reduction. Rapamycin-loaded immunoliposomes were prepared with high trastuzumab functionalization efficiency and antibody stability. Cytotoxicity studies showed that the HER2-positive SK-BR-3 cell line was sensitive to trastuzumab, either as free drug or in the context of immunoliposomes, and is more sensitive to rapamycin than the triple negative MDA-MB-231 cells. For MDA-MB-231, the liposomal rapamycin was more cytotoxic than the free drug. Furthermore, the immunoliposomes showed potent cytotoxicity against SK-BR-3 cells. Finally, rapamycin and trastuzumab exhibited in vitro synergistic effect, particularly through immunoliposomes.
Conclusion: The formulation developed herein has potential for in vivo evaluation.
Figures






References
-
- Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM, Ferrari M. Nanotechnology for breast cancer therapy. Biomed. Microdevices. 2009;11:49–63. - PubMed
-
- Engel RH, Kaklamani VG. HER2-positive breast cancer: Current and future treatment strategies. Drugs. 2007;67:1329–1341. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

