Pharmacological Amelioration of Cone Survival and Vision in a Mouse Model for Leber Congenital Amaurosis
- PMID: 27225770
- PMCID: PMC4879198
- DOI: 10.1523/JNEUROSCI.3857-15.2016
Pharmacological Amelioration of Cone Survival and Vision in a Mouse Model for Leber Congenital Amaurosis
Abstract
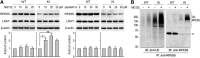
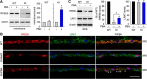
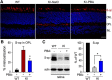
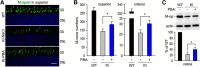
RPE65, an abundant membrane-associate protein in the retinal pigment epithelium (RPE), is a key retinoid isomerase of the visual cycle necessary for generating 11-cis-retinal that functions not only as a molecular switch for activating cone and rod visual pigments in response to light stimulation, but also as a chaperone for normal trafficking of cone opsins to the outer segments. Many mutations in RPE65 are associated with Leber congenital amaurosis (LCA). A R91W substitution, the most frequent LCA-associated mutation, results in a severe decrease in protein level and enzymatic activity of RPE65, causing cone opsin mislocalization and early cone degeneration in the mutation knock-in mouse model of LCA. Here we show that R91W RPE65 undergoes ubiquitination-dependent proteasomal degradation in the knock-in mouse RPE due to misfolding. The 26S proteasome non-ATPase regulatory subunit 13 mediated degradation specifically of misfolded R91W RPE65. The mutation disrupted membrane-association and colocalization of RPE65 with lecithin:retinol acyltransferase (LRAT) that provides the hydrophobic substrate for RPE65. Systemic administration of sodium 4-phenylbutyrate (PBA), a chemical chaperone, increased protein stability, enzymatic activity, membrane-association, and colocalization of R91W RPE65 with LRAT. This rescue effect increased synthesis of 11-cis-retinal and 9-cis-retinal, a functional iso-chromophore of the visual pigments, led to alleviation of S-opsin mislocalization and cone degeneration in the knock-in mice. Importantly, PBA-treatment also improved cone-mediated vision in the mutant mice. These results indicate that PBA, a U.S. Food and Drug Administration-approved safe oral medication, may provide a noninvasive therapeutic intervention that delays daylight vision loss in patients with RPE65 mutations.
Significance statement: LCA is a severe early onset retinal dystrophy. Recent clinical trials of gene therapy have implicated the need of an alternative or combination therapy to improve cone survival and function in patients with LCA caused by RPE65 mutations. Using a mouse model carrying the most frequent LCA-associated mutation (R91W), we found that the mutant RPE65 underwent ubiquitination-dependent proteasomal degradation due to misfolding. Treatment of the mice with a chemical chaperone partially corrected stability, enzymatic activity, and subcellular localization of R91W RPE65, which was also accompanied by improvement of cone survival and vision. These findings identify an in vivo molecular pathogenic mechanism for R91W mutation and provide a feasible pharmacological approach that can delay vision loss in patients with RPE65 mutations.
Keywords: Leber congenital amaurosis; Rpe65; chemical chaperone; cone photoreceptor; retina; retinoid visual cycle.
Copyright © 2016 the authors 0270-6474/16/365808-12$15.00/0.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Intraperitoneal chromophore injections delay early-onset and rapid retinal cone degeneration in a mouse model of Leber congenital amaurosis.Exp Eye Res. 2021 Nov;212:108776. doi: 10.1016/j.exer.2021.108776. Epub 2021 Sep 25. Exp Eye Res. 2021. PMID: 34582935
-
Inverse correlation between fatty acid transport protein 4 and vision in Leber congenital amaurosis associated with RPE65 mutation.Proc Natl Acad Sci U S A. 2020 Dec 15;117(50):32114-32123. doi: 10.1073/pnas.2012623117. Epub 2020 Nov 30. Proc Natl Acad Sci U S A. 2020. PMID: 33257550 Free PMC article.
-
Ablation of Fatty Acid Transport Protein-4 Enhances Cone Survival, M-cone Vision, and Synthesis of Cone-Tropic 9-cis-Retinal in rd12 Mouse Model of Leber Congenital Amaurosis.J Neurosci. 2024 Jul 3;44(27):e1994232024. doi: 10.1523/JNEUROSCI.1994-23.2024. J Neurosci. 2024. PMID: 38811164 Free PMC article.
-
Cone-rod dystrophy caused by a novel homozygous RPE65 mutation in Leber congenital amaurosis.Klin Monbl Augenheilkd. 2014 Apr;231(4):405-10. doi: 10.1055/s-0034-1368221. Epub 2014 Apr 25. Klin Monbl Augenheilkd. 2014. PMID: 24771178 Review.
-
Gene therapy for Leber congenital amaurosis: advances and future directions.Graefes Arch Clin Exp Ophthalmol. 2012 Aug;250(8):1117-28. doi: 10.1007/s00417-012-2028-2. Epub 2012 May 29. Graefes Arch Clin Exp Ophthalmol. 2012. PMID: 22644094 Free PMC article. Review.
Cited by
-
Pathomechanisms of Inherited Retinal Degeneration and Perspectives for Neuroprotection.Cold Spring Harb Perspect Med. 2023 Jun 1;13(6):a041310. doi: 10.1101/cshperspect.a041310. Cold Spring Harb Perspect Med. 2023. PMID: 36122932 Free PMC article. Review.
-
Increased ER Stress After Experimental Ischemic Optic Neuropathy and Improved RGC and Oligodendrocyte Survival After Treatment With Chemical Chaperon.Invest Ophthalmol Vis Sci. 2019 May 1;60(6):1953-1966. doi: 10.1167/iovs.18-24890. Invest Ophthalmol Vis Sci. 2019. PMID: 31060051 Free PMC article.
-
Drug Discovery Strategies for Inherited Retinal Degenerations.Biology (Basel). 2022 Sep 10;11(9):1338. doi: 10.3390/biology11091338. Biology (Basel). 2022. PMID: 36138817 Free PMC article. Review.
-
Evaluation for Retinal Therapy for RPE65 Variation Assessed in hiPSC Retinal Pigment Epithelial Cells.Stem Cells Int. 2021 Dec 13;2021:4536382. doi: 10.1155/2021/4536382. eCollection 2021. Stem Cells Int. 2021. PMID: 34938339 Free PMC article.
-
Neuroprotective Effect of 4-Phenylbutyric Acid against Photo-Stress in the Retina.Antioxidants (Basel). 2021 Jul 20;10(7):1147. doi: 10.3390/antiox10071147. Antioxidants (Basel). 2021. PMID: 34356380 Free PMC article.
References
-
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. - DOI - PubMed
-
- Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ, Feathers KL, Luong VA, Yzer S, Balaggan K, Viswanathan A, de Ravel TJ, Casteels I, Holder GE, Tyler N, Fitzke FW, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. - DOI - PMC - PubMed
-
- Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
