NLRP3 Deficiency Reduces Macrophage Interleukin-10 Production and Enhances the Susceptibility to Doxorubicin-induced Cardiotoxicity
- PMID: 27225830
- PMCID: PMC4880937
- DOI: 10.1038/srep26489
NLRP3 Deficiency Reduces Macrophage Interleukin-10 Production and Enhances the Susceptibility to Doxorubicin-induced Cardiotoxicity
Abstract
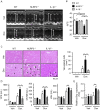
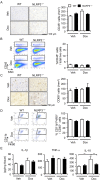
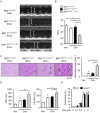
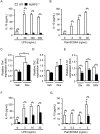
NLRP3 inflammasomes recognize non-microbial danger signals and induce release of proinflammatory cytokine interleukin (IL)-1β, leading to sterile inflammation in cardiovascular disease. Because sterile inflammation is involved in doxorubicin (Dox)-induced cardiotoxicity, we investigated the role of NLRP3 inflammasomes in Dox-induced cardiotoxicity. Cardiac dysfunction and injury were induced by low-dose Dox (15 mg/kg) administration in NLRP3-deficient (NLRP3(-/-)) mice but not in wild-type (WT) and IL-1β(-/-) mice, indicating that NLRP3 deficiency enhanced the susceptibility to Dox-induced cardiotoxicity independent of IL-1β. Although the hearts of WT and NLRP3(-/-) mice showed no significant difference in inflammatory cell infiltration, macrophages were the predominant inflammatory cells in the hearts, and cardiac IL-10 production was decreased in Dox-treated NLRP3(-/-) mice. Bone marrow transplantation experiments showed that bone marrow-derived cells contributed to the exacerbation of Dox-induced cardiotoxicity in NLRP3(-/-) mice. In vitro experiments revealed that NLRP3 deficiency decreased IL-10 production in macrophages. Furthermore, adeno-associated virus-mediated IL-10 overexpression restored the exacerbation of cardiotoxicity in the NLRP3(-/-) mice. These results demonstrated that NLRP3 regulates macrophage IL-10 production and contributes to the pathophysiology of Dox-induced cardiotoxicity, which is independent of IL-1β. Our findings identify a novel role of NLRP3 and provided new insights into the mechanisms underlying Dox-induced cardiotoxicity.
Figures
Similar articles
-
The beneficial effects of reducing NLRP3 inflammasome activation in the cardiotoxicity and the anti-cancer effects of doxorubicin.Arch Toxicol. 2021 Jan;95(1):1-9. doi: 10.1007/s00204-020-02876-2. Epub 2020 Aug 27. Arch Toxicol. 2021. PMID: 32852568 Review.
-
The cardiac glycoside ouabain activates NLRP3 inflammasomes and promotes cardiac inflammation and dysfunction.PLoS One. 2017 May 11;12(5):e0176676. doi: 10.1371/journal.pone.0176676. eCollection 2017. PLoS One. 2017. PMID: 28493895 Free PMC article.
-
NLRP3 inflammasome mediates interleukin-1β production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice.Immunology. 2017 Apr;150(4):495-505. doi: 10.1111/imm.12704. Epub 2017 Jan 30. Immunology. 2017. PMID: 28032341 Free PMC article.
-
The Role of Cytokines Produced via the NLRP3 Inflammasome in Mouse Macrophages Stimulated with Dental Calculus in Osteoclastogenesis.Int J Mol Sci. 2021 Nov 18;22(22):12434. doi: 10.3390/ijms222212434. Int J Mol Sci. 2021. PMID: 34830316 Free PMC article.
-
NLRP3 inflammasome as a therapeutic target in doxorubicin-induced cardiotoxicity: role of phytochemicals.Front Pharmacol. 2025 Apr 17;16:1567312. doi: 10.3389/fphar.2025.1567312. eCollection 2025. Front Pharmacol. 2025. PMID: 40313623 Free PMC article. Review.
Cited by
-
The beneficial effects of reducing NLRP3 inflammasome activation in the cardiotoxicity and the anti-cancer effects of doxorubicin.Arch Toxicol. 2021 Jan;95(1):1-9. doi: 10.1007/s00204-020-02876-2. Epub 2020 Aug 27. Arch Toxicol. 2021. PMID: 32852568 Review.
-
Glabridin Prevents Doxorubicin-Induced Cardiotoxicity Through Gut Microbiota Modulation and Colonic Macrophage Polarization in Mice.Front Pharmacol. 2019 Feb 15;10:107. doi: 10.3389/fphar.2019.00107. eCollection 2019. Front Pharmacol. 2019. PMID: 30833897 Free PMC article.
-
Vitamin D3 attenuates doxorubicin-induced senescence of human aortic endothelial cells by upregulation of IL-10 via the pAMPKα/Sirt1/Foxo3a signaling pathway.PLoS One. 2021 Jun 8;16(6):e0252816. doi: 10.1371/journal.pone.0252816. eCollection 2021. PLoS One. 2021. PMID: 34101754 Free PMC article.
-
Relevance of Caspase-1 and Nlrp3 Inflammasome on Inflammatory Bone Resorption in A Murine Model of Periodontitis.Sci Rep. 2020 May 8;10(1):7823. doi: 10.1038/s41598-020-64685-y. Sci Rep. 2020. PMID: 32385413 Free PMC article.
-
Adeno-associated Virus Vector-mediated Interleukin-10 Induction Prevents Vascular Inflammation in a Murine Model of Kawasaki Disease.Sci Rep. 2018 May 15;8(1):7601. doi: 10.1038/s41598-018-25856-0. Sci Rep. 2018. PMID: 29765083 Free PMC article.
References
-
- Singal P. K. & Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 339, 900–905 (1998). - PubMed
-
- Octavia Y. et al. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52, 1213–1225 (2012). - PubMed
-
- Takemura G. & Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49, 330–352 (2007). - PubMed
-
- Riad A. et al. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail 10, 233–243 (2008). - PubMed
-
- Nozaki N., Shishido T., Takeishi Y. & Kubota I. Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation 110, 2869–2874 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

