Prenatal ketamine exposure causes abnormal development of prefrontal cortex in rat
- PMID: 27226073
- PMCID: PMC4881038
- DOI: 10.1038/srep26865
Prenatal ketamine exposure causes abnormal development of prefrontal cortex in rat
Abstract
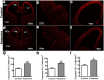
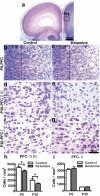
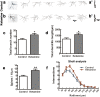
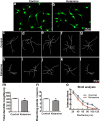
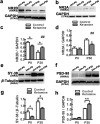
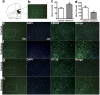
Ketamine is commonly used for anesthesia and as a recreational drug. In pregnant users, a potential neurotoxicity in offspring has been noted. Our previous work demonstrated that ketamine exposure of pregnant rats induces affective disorders and cognitive impairments in offspring. As the prefrontal cortex (PFC) is critically involved in emotional and cognitive processes, here we studied whether maternal ketamine exposure influences the development of the PFC in offspring. Pregnant rats on gestational day 14 were treated with ketamine at a sedative dose for 2 hrs, and pups were studied at postnatal day 0 (P0) or P30. We found that maternal ketamine exposure resulted in cell apoptosis and neuronal loss in fetal brain. Upon ketamine exposure in utero, PFC neurons at P30 showed more dendritic branching, while cultured neurons from P0 PFC extended shorter neurites than controls. In addition, maternal ketamine exposure postponed the switch of NR2B/2A expression, and perturbed pre- and postsynaptic protein expression in the PFC. These data suggest that prenatal ketamine exposure impairs neuronal development of the PFC, which may be associated with abnormal behavior in offsprings.
Figures


Similar articles
-
Ketamine administered to pregnant rats in the second trimester causes long-lasting behavioral disorders in offspring.Neurobiol Dis. 2014 Aug;68:145-55. doi: 10.1016/j.nbd.2014.02.009. Epub 2014 Apr 26. Neurobiol Dis. 2014. PMID: 24780497
-
17β-estradiol attenuates ketamine-induced neuroapoptosis and persistent cognitive deficits in the developing brain.Brain Res. 2014 Dec 17;1593:30-9. doi: 10.1016/j.brainres.2014.09.013. Epub 2014 Sep 16. Brain Res. 2014. PMID: 25234726
-
Embryonic Ketamine Produces a Downregulation of Prefrontal Cortex NMDA Receptors and Anxiety-Like Behavior in Adult Offspring.Neuroscience. 2019 Sep 1;415:18-30. doi: 10.1016/j.neuroscience.2019.07.018. Epub 2019 Jul 17. Neuroscience. 2019. PMID: 31325561
-
Relationship between ketamine-induced developmental neurotoxicity and NMDA receptor-mediated calcium influx in neural stem cell-derived neurons.Neurotoxicology. 2017 May;60:254-259. doi: 10.1016/j.neuro.2016.04.015. Epub 2016 Apr 27. Neurotoxicology. 2017. PMID: 27132109 Review.
-
Dual effects of ketamine: neurotoxicity versus neuroprotection in anesthesia for the developing brain.J Neurosurg Anesthesiol. 2014 Apr;26(2):155-60. doi: 10.1097/ANA.0000000000000027. J Neurosurg Anesthesiol. 2014. PMID: 24275940 Review.
Cited by
-
Ketamine impairs growth cone and synaptogenesis in human GABAergic projection neurons via GSK-3β and HDAC6 signaling.Mol Psychiatry. 2024 Jun;29(6):1647-1659. doi: 10.1038/s41380-022-01864-5. Epub 2022 Nov 21. Mol Psychiatry. 2024. PMID: 36414713 Free PMC article.
-
Ex Utero Electroporation and Organotypic Slice Cultures of Embryonic Mouse Brains for Live-Imaging of Migrating GABAergic Interneurons.J Vis Exp. 2018 Apr 20;(134):57526. doi: 10.3791/57526. J Vis Exp. 2018. PMID: 29733310 Free PMC article.
-
Glutamate Deregulation in Ketamine-Induced Psychosis-A Potential Role of PSD95, NMDA Receptor and PMCA Interaction.Front Cell Neurosci. 2017 Jun 28;11:181. doi: 10.3389/fncel.2017.00181. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28701926 Free PMC article.
-
Risks Associated with Misuse of Ketamine as a Rapid-Acting Antidepressant.Neurosci Bull. 2016 Dec;32(6):557-564. doi: 10.1007/s12264-016-0081-2. Epub 2016 Nov 22. Neurosci Bull. 2016. PMID: 27878517 Free PMC article. Review.
-
In vivo two-photon imaging of the embryonic cortex reveals spontaneous ketamine-sensitive calcium activity.Sci Rep. 2018 Oct 30;8(1):16059. doi: 10.1038/s41598-018-34410-x. Sci Rep. 2018. PMID: 30375447 Free PMC article.
References
-
- Craven R. Ketamine. Anaesthesia 62 Suppl 1, 48–53 (2007). - PubMed
-
- Cheek T. G. & Baird E. Anesthesia for nonobstetric surgery: maternal and fetal considerations. Clin Obstet Gynecol 52, 535–545 (2009). - PubMed
-
- Bokor G. & Anderson P. D. Ketamine: an update on its abuse. J Pharm Pract 27, 582–586 (2014). - PubMed
-
- Degenhardt L. & Dunn M. The epidemiology of GHB and ketamine use in an Australian household survey. Int J Drug Policy 19, 311–316 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

