A review of disease progression models of Parkinson's disease and applications in clinical trials
- PMID: 27226141
- PMCID: PMC4931998
- DOI: 10.1002/mds.26644
A review of disease progression models of Parkinson's disease and applications in clinical trials
Abstract
Quantitative disease progression models for neurodegenerative disorders are gaining recognition as important tools for drug development and evaluation. In Parkinson's disease (PD), several models have described longitudinal changes in the Unified Parkinson's Disease Rating Scale (UPDRS), one of the most utilized outcome measures for PD trials assessing disease progression. We conducted a literature review to examine the methods and applications of quantitative disease progression modeling for PD using a combination of key words including "Parkinson disease," "progression," and "model." For this review, we focused on models of PD progression quantifying changes in the total UPDRS scores against time. Four different models reporting equations and parameters have been published using linear and nonlinear functions. The reasons for constructing disease progression models of PD thus far have been to quantify disease trajectories of PD patients in active and inactive treatment arms of clinical trials, to quantify and discern symptomatic and disease-modifying treatment effects, and to demonstrate how model-based methods may be used to design clinical trials. The historical lack of efficiency of PD clinical trials begs for model-based simulations in planning for studies that result in more informative conclusions, particularly around disease modification. © 2016 International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease; UPDRS; disease model; disease progression; pharmacometrics.
© 2016 International Parkinson and Movement Disorder Society.
Figures
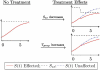
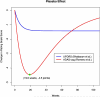
References
-
- National Institute of Neurological Disorders and Stroke Parkinson's disease 2014 advancing research improving lives conference recommendations report to the National Advisory Neurological Disorders and Stroke Council. 2014 Jan 30; Retrieved from http://www.ninds.nih.gov/research/parkinsonsweb/PD2014/
-
- Mould DR, Denman NG, Dufull S. Using disease progression models as a tool to detect drug effect. Clin Pharmacol Ther. 2007;82(1):81–6. - PubMed
-
- Samtani MN, Raghavan N, Shi Y, et al. Alzheimer's Disease Neuroimaging Initiative Disease progression model in subjects with mild cognitive impairment from the Alzheimer's disease neuroimaging initiative: CSF biomarkers predict population subtypes. Br J Clin Pharmacol. 2013;75(1):146–61. - PMC - PubMed
-
- Passey C, Kimko H, Nandy P, Kagan L. Osteoarthritis disease progression model using six year follow-up data from the osteoarthritis initiative. J Clin Pharmacol. 2014 doi: 10.1002/jcph.399. [Epub ahead of print] - PubMed
-
- Atchison TB, Massman PJ, Doody RS. Baseline cognitive function predicts rate of decline in basic-care abilities of individuals with dementia of the Alzheimer's type. Arch Clin Neuropsychol. 2007;22(1):99–107. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

