A system for studying mechanisms of neuromuscular junction development and maintenance
- PMID: 27226316
- PMCID: PMC4958317
- DOI: 10.1242/dev.130278
A system for studying mechanisms of neuromuscular junction development and maintenance
Abstract
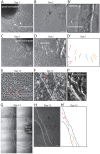
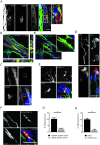
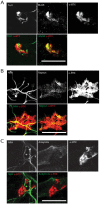
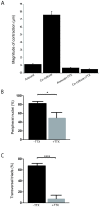
The neuromuscular junction (NMJ), a cellular synapse between a motor neuron and a skeletal muscle fiber, enables the translation of chemical cues into physical activity. The development of this special structure has been subject to numerous investigations, but its complexity renders in vivo studies particularly difficult to perform. In vitro modeling of the neuromuscular junction represents a powerful tool to delineate fully the fine tuning of events that lead to subcellular specialization at the pre-synaptic and post-synaptic sites. Here, we describe a novel heterologous co-culture in vitro method using rat spinal cord explants with dorsal root ganglia and murine primary myoblasts to study neuromuscular junctions. This system allows the formation and long-term survival of highly differentiated myofibers, motor neurons, supporting glial cells and functional neuromuscular junctions with post-synaptic specialization. Therefore, fundamental aspects of NMJ formation and maintenance can be studied using the described system, which can be adapted to model multiple NMJ-associated disorders.
Keywords: Co-culture; Differentiation; Myofiber; NMJ.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




References
-
- Achtstätter T., Moll R., Anderson A., Kuhn C., Pitz S., Schwechheimer K. and Franke W. W. (1986). Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation 31, 206-227. 10.1111/j.1432-0436.1986.tb00401.x - DOI - PubMed
-
- Arnold A.-S., Christe M. and Handschin C. (2012). A functional motor unit in the culture dish: co-culture of spinal cord explants and muscle cells. J. Vis. Exp. 62, doi:10.3791/3616 10.3791/3616 - DOI - PMC - PubMed
-
- Askanas V., Kwan H., Alvarez R. B., Engel W. K., Kobayashi T., Martinuzzi A. and Hawkins E. F. (1987). De novo neuromuscular junction formation on human muscle fibres cultured in monolayer and innervated by foetal rat spinal cord: ultrastructural and ultrastructural-cytochemical studies. J. Neurocytol. 16, 523-537. 10.1007/BF01668506 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

