HIV-1-Specific Chimeric Antigen Receptors Based on Broadly Neutralizing Antibodies
- PMID: 27226366
- PMCID: PMC4944295
- DOI: 10.1128/JVI.00805-16
HIV-1-Specific Chimeric Antigen Receptors Based on Broadly Neutralizing Antibodies
Abstract
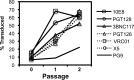
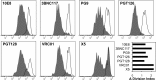
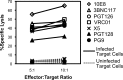
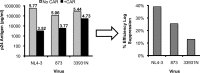
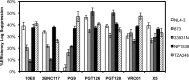
Although the use of chimeric antigen receptors (CARs) based on single-chain antibodies for gene immunotherapy of cancers is increasing due to promising recent results, the earliest CAR therapeutic trials were done for HIV-1 infection in the late 1990s. This approach utilized a CAR based on human CD4 as a binding domain and was abandoned for a lack of efficacy. The growing number of HIV-1 broadly neutralizing antibodies (BNAbs) offers the opportunity to generate novel CARs that may be more active and revisit this modality for HIV-1 immunotherapy. We used sequences from seven well-defined BNAbs varying in binding sites and generated single-chain-antibody-based CARs. These CARs included 10E8, 3BNC117, PG9, PGT126, PGT128, VRC01, and X5. Each novel CAR exhibited conformationally relevant expression on the surface of transduced cells, mediated specific proliferation and killing in response to HIV-1-infected cells, and conferred potent antiviral activity (reduction of viral replication in log10 units) to transduced CD8(+) T lymphocytes. The antiviral activity of these CARs was reproducible but varied according to the strain of virus. These findings indicated that BNAbs are excellent candidates for developing novel CARs to consider for the immunotherapeutic treatment of HIV-1.
Importance: While chimeric antigen receptors (CARs) using single-chain antibodies as binding domains are growing in popularity for gene immunotherapy of cancers, the earliest human trials of CARs were done for HIV-1 infection. However, those trials failed, and the approach was abandoned for HIV-1. The only tested CAR against HIV-1 was based on the use of CD4 as the binding domain. The growing availability of HIV-1 broadly neutralizing antibodies (BNAbs) affords the opportunity to revisit gene immunotherapy for HIV-1 using novel CARs based on single-chain antibodies. Here we construct and test a panel of seven novel CARs based on diverse BNAb types and show that all these CARs are functional against HIV-1.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Roberts MR, Qin L, Zhang D, Smith DH, Tran AC, Dull TJ, Groopman JE, Capon DJ, Byrn RA, Finer MH. 1994. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 84:2878–2889. - PubMed
-
- Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, Bakker A, Roberts MR, June CH, Jalali S, Lin AA, Pennathur-Das R, Hege KM. 2000. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood 96:785–793. - PubMed
-
- Deeks SG, Wagner B, Anton PA, Mitsuyasu RT, Scadden DT, Huang C, Macken C, Richman DD, Christopherson C, June CH, Lazar R, Broad DF, Jalali S, Hege KM. 2002. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol Ther 5:788–797. doi: 10.1006/mthe.2002.0611. - DOI - PubMed
-
- Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH. 2012. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 4:132ra53. doi: 10.1126/scitranslmed.3003761. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

