Thyroid Hormone Receptor β Agonist Induces β-Catenin-Dependent Hepatocyte Proliferation in Mice: Implications in Hepatic Regeneration
- PMID: 27226410
- PMCID: PMC5215473
- DOI: 10.3727/105221616X691631
Thyroid Hormone Receptor β Agonist Induces β-Catenin-Dependent Hepatocyte Proliferation in Mice: Implications in Hepatic Regeneration
Abstract
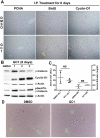
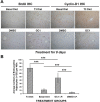
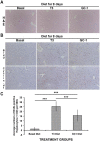
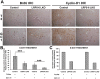
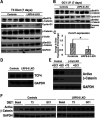
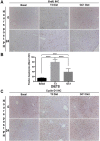
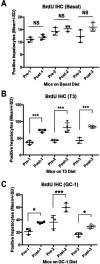
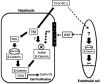
Triiodothyronine (T3) induces hepatocyte proliferation in rodents. Recent work has shown molecular mechanism for T3's mitogenic effect to be through activation of β-catenin signaling. Since systemic side effects of T3 may preclude its clinical use, and hepatocytes mostly express T3 hormone receptor β (TRβ), we investigated if selective TRβ agonists like GC-1 may also have β-catenin-dependent hepatocyte mitogenic effects. Here we studied the effect of GC-1 and T3 in conditional knockouts of various Wnt pathway components. We also assessed any regenerative advantage of T3 or GC-1 when given prior to partial hepatectomy in mice. Mice administered GC-1 showed increased pSer675-β-catenin, cyclin D1, BrdU incorporation, and PCNA. No abnormalities in liver function tests were noted. GC-1-injected liver-specific β-catenin knockouts (β-catenin LKO) showed decreased proliferation when compared to wild-type littermates. To address if Wnt signaling was required for T3- or GC-1-mediated hepatocyte proliferation, we used LRP5-6-LKO, which lacks the two redundant Wnt coreceptors. Surprisingly, decreased hepatocyte proliferation was also evident in LRP5-6-LKO in response to T3 and GC-1, despite increased pSer675-β-catenin. Further, increased levels of active β-catenin (hypophosphorylated at Ser33, Ser37, and Thr41) were evident after T3 and GC-1 treatment. Finally, mice pretreated with T3 or GC-1 for 7 days followed by partial hepatectomy showed a significant increase in hepatocyte proliferation both at the time (T0) and 24 h after surgery. In conclusion, like T3, TRβ-selective agonists induce hepatocyte proliferation through β-catenin activation via both PKA- and Wnt-dependent mechanisms and confer a regenerative advantage following surgical resection. Hence, these agents may be useful regenerative therapies in liver transplantation or other surgical settings.
Figures

References
-
- Karp SJ. Clinical implications of advances in the basic science of liver repair and regeneration. Am J Transplant. 2009;9:1973–80. - PubMed
-
- Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis AP, Puder M, Clevers H, Moon RT, Zon LI. APC mutant zebrafish uncover a changing temporal requirement for Wnt signaling in liver development. Dev Biol. 2008;320:161–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
