Stress Degrades Prefrontal Cortex Neuronal Coding of Goal-Directed Behavior
- PMID: 27226444
- PMCID: PMC6059199
- DOI: 10.1093/cercor/bhw140
Stress Degrades Prefrontal Cortex Neuronal Coding of Goal-Directed Behavior
Abstract
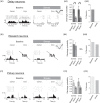
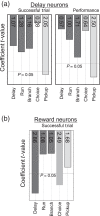
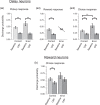
Stress, pervasive in modern society, impairs prefrontal cortex (PFC)-dependent cognitive processes, an action implicated in multiple psychopathologies and estimated to contribute to nearly half of all work place accidents. However, the neurophysiological bases for stress-related impairment of PFC-dependent function remain poorly understood. The current studies examined the effects of stress on PFC neural coding during a working memory task in rats. Stress suppressed responses of medial PFC (mPFC) neurons strongly tuned to a diversity of task events, including delay and outcome (reward, error). Stress-related impairment of task-related neuronal activity included multidimensional coding by PFC neurons, an action that significantly predicted cognitive impairment. Importantly, the effects of stress on PFC neuronal signaling were highly conditional on tuning strength: stress increased task-related activity in the larger population of PFC neurons weakly tuned to task events. Combined, stress elicits a profound collapse of task representations across the broader population of PFC neurons.
Keywords: attention; cognition; multichannel recording; outcome-evaluation; prefrontal cortex; reward; working memory.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



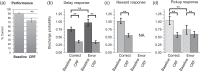

References
-
- Arnsten AF, Goldman-Rakic PS. 1998. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 55:362–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

