Respiratory and autonomic dysfunction in congenital central hypoventilation syndrome
- PMID: 27226447
- PMCID: PMC6208311
- DOI: 10.1152/jn.00026.2016
Respiratory and autonomic dysfunction in congenital central hypoventilation syndrome
Abstract
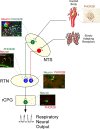
The developmental lineage of the PHOX2B-expressing neurons in the retrotrapezoid nucleus (RTN) has been extensively studied. These cells are thought to function as central respiratory chemoreceptors, i.e., the mechanism by which brain Pco2 regulates breathing. The molecular and cellular basis of central respiratory chemoreception is based on the detection of CO2 via intrinsic proton receptors (TASK-2, GPR4) as well as synaptic input from peripheral chemoreceptors and other brain regions. Murine models of congenital central hypoventilation syndrome designed with PHOX2B mutations have suggested RTN neuron agenesis. In this review, we examine, through human and experimental animal models, how a restricted number of neurons that express the transcription factor PHOX2B play a crucial role in the control of breathing and autonomic regulation.
Keywords: CCHS; breathing; chemoreflex; ventrolateral medulla.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB 3rd. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development : 4449–4461, 2001. - PubMed
-
- Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol : 125–132, 2009. - PubMed
-
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet : 459–461, 2003. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

