Rhythmic modulation of visual contrast discrimination triggered by action
- PMID: 27226468
- PMCID: PMC4892807
- DOI: 10.1098/rspb.2016.0692
Rhythmic modulation of visual contrast discrimination triggered by action
Abstract
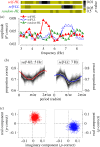
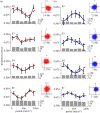
Recent evidence suggests that ongoing brain oscillations may be instrumental in binding and integrating multisensory signals. In this experiment, we investigated the temporal dynamics of visual-motor integration processes. We show that action modulates sensitivity to visual contrast discrimination in a rhythmic fashion at frequencies of about 5 Hz (in the theta range), for up to 1 s after execution of action. To understand the origin of the oscillations, we measured oscillations in contrast sensitivity at different levels of luminance, which is known to affect the endogenous brain rhythms, boosting the power of alpha-frequencies. We found that the frequency of oscillation in sensitivity increased at low luminance, probably reflecting the shift in mean endogenous brain rhythm towards higher frequencies. Importantly, both at high and at low luminance, contrast discrimination showed a rhythmic motor-induced suppression effect, with the suppression occurring earlier at low luminance. We suggest that oscillations play a key role in sensory-motor integration, and that the motor-induced suppression may reflect the first manifestation of a rhythmic oscillation.
Keywords: action and perception; contrast discrimination; endogenous rhythm; sensory–motor integration; visual oscillations.
© 2016 The Author(s).
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
