Genetic Ablation of Calcium-independent Phospholipase A2γ Induces Glomerular Injury in Mice
- PMID: 27226532
- PMCID: PMC4938171
- DOI: 10.1074/jbc.M115.696781
Genetic Ablation of Calcium-independent Phospholipase A2γ Induces Glomerular Injury in Mice
Abstract
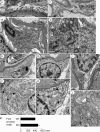
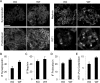
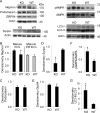
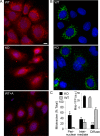
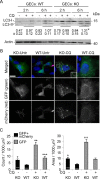
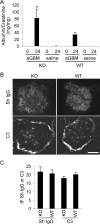
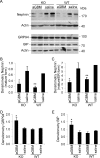
Glomerular visceral epithelial cells (podocytes) play a critical role in the maintenance of glomerular permselectivity. Podocyte injury, manifesting as proteinuria, is the cause of many glomerular diseases. We reported previously that calcium-independent phospholipase A2γ (iPLA2γ) is cytoprotective against complement-mediated glomerular epithelial cell injury. Studies in iPLA2γ KO mice have demonstrated an important role for iPLA2γ in mitochondrial lipid turnover, membrane structure, and metabolism. The aim of the present study was to employ iPLA2γ KO mice to better understand the role of iPLA2γ in normal glomerular and podocyte function as well as in glomerular injury. We show that deletion of iPLA2γ did not cause detectable albuminuria; however, it resulted in mitochondrial structural abnormalities and enhanced autophagy in podocytes as well as loss of podocytes in aging KO mice. Moreover, after induction of anti-glomerular basement membrane nephritis in young mice, iPLA2γ KO mice exhibited significantly increased levels of albuminuria, podocyte injury, and loss of podocytes compared with wild type. Thus, iPLA2γ has a protective functional role in the normal glomerulus and in glomerulonephritis. Understanding the role of iPLA2γ in glomerular pathophysiology provides opportunities for the development of novel therapeutic approaches to glomerular injury and proteinuria.
Keywords: Phospholipase A; autophagy; cell injury; glomerulonephritis; mitochondria; podocyte; proteinuria.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Murakami M., and Kudo I. (2002) Phospholipase A2. J. Biochem. 131, 285–292 - PubMed
-
- Six D. A., and Dennis E. A. (2000) The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta 1488, 1–19 - PubMed
-
- Schaloske R. H., and Dennis E. A. (2006) The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 1761, 1246–1259 - PubMed
-
- Mancuso D. J., Jenkins C. M., and Gross R. W. (2000) The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A2. J. Biol. Chem. 275, 9937–9945 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

