Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease
- PMID: 27226559
- PMCID: PMC4946939
- DOI: 10.1074/jbc.M116.714824
Neutralization of RANTES and Eotaxin Prevents the Loss of Dopaminergic Neurons in a Mouse Model of Parkinson Disease
Retraction in
-
Withdrawal: Neutralization of RANTES and eotaxin prevents the loss of dopaminergic neurons in a mouse model of Parkinson disease.J Biol Chem. 2025 Jan;301(1):108102. doi: 10.1016/j.jbc.2024.108102. Epub 2025 Jan 9. J Biol Chem. 2025. PMID: 39793405 Free PMC article. No abstract available.
Abstract
Parkinson disease (PD) is second only to Alzheimer disease as the most common human neurodegenerative disorder. Despite intense investigation, no interdictive therapy is available for PD. Recent studies indicate that both innate and adaptive immune processes are active in PD. Accordingly, we found a rapid increase in RANTES (regulated on activation normal T cell expressed and secreted) and eotaxin, chemokines that are involved in T cell trafficking, in vivo in the substantia nigra pars compacta and the serum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-intoxicated mice. RANTES and eotaxin were also up-regulated in the substantia nigra pars compacta of post-mortem PD brains as compared with age-matched controls. Therefore, we investigated whether neutralization of RANTES and eotaxin could protect against nigrostriatal degeneration in MPTP-intoxicated mice. Interestingly, after peripheral administration, functional blocking antibodies against RANTES and eotaxin reduced the infiltration of CD4(+) and CD8(+) T cells into the nigra, attenuated nigral expression of proinflammatory molecules, and suppressed nigral activation of glial cells. These findings paralleled dopaminergic neuronal protection, normalized striatal neurotransmitters, and improved motor functions in MPTP-intoxicated mice. Therefore, we conclude that attenuation of the chemokine-dependent adaptive immune response may be of therapeutic benefit for PD patients.
Keywords: Parkinson disease; T-cell; animal model; glial cell; neurodegeneration; neuroinflammation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
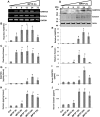
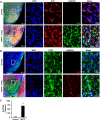
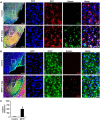

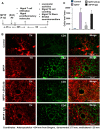
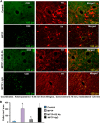
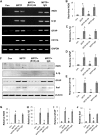
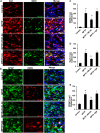
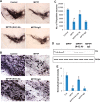
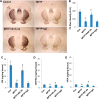
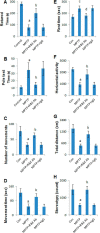
References
-
- Vila M., and Przedborski S. (2004) Genetic clues to the pathogenesis of Parkinson's disease. Nat. Med. 10, (suppl.) S58–S62 - PubMed
-
- Olanow C. W., and Tatton W. G. (1999) Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 22, 123–144 - PubMed
-
- Dauer W., and Przedborski S. (2003) Parkinson's disease: mechanisms and models. Neuron 39, 889–909 - PubMed
-
- Brochard V., Combadière B., Prigent A., Laouar Y., Perrin A., Beray-Berthat V., Bonduelle O., Alvarez-Fischer D., Callebert J., Launay J. M., Duyckaerts C., Flavell R. A., Hirsch E. C., and Hunot S. (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 119, 182–192 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

