How Phosphorylation and ATPase Activity Regulate Anion Flux though the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
- PMID: 27226582
- PMCID: PMC4938172
- DOI: 10.1074/jbc.M116.721415
How Phosphorylation and ATPase Activity Regulate Anion Flux though the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR)
Erratum in
-
How phosphorylation and ATPase activity regulate anion flux through the cystic fibrosis transmembrane conductance regulator (CFTR).J Biol Chem. 2016 Nov 11;291(46):23928. doi: 10.1074/jbc.A116.721415. J Biol Chem. 2016. PMID: 27837011 Free PMC article. No abstract available.
Abstract
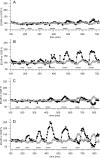
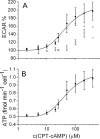
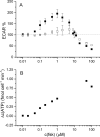
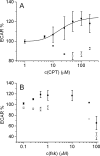
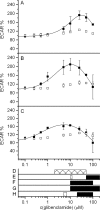
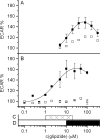
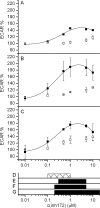
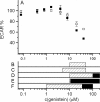
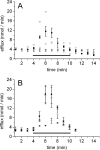
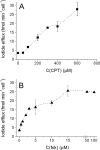
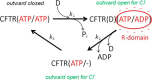
The cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7), mutations of which cause cystic fibrosis, belongs to the ATP-binding cassette (ABC) transporter family and works as a channel for small anions, such as chloride and bicarbonate. Anion channel activity is known to depend on phosphorylation by cAMP-dependent protein kinase A (PKA) and CFTR-ATPase activity. Whereas anion channel activity has been extensively investigated, phosphorylation and CFTR-ATPase activity are still poorly understood. Here, we show that the two processes can be measured in a label-free and non-invasive manner in real time in live cells, stably transfected with CFTR. This study reveals three key findings. (i) The major contribution (≥90%) to the total CFTR-related ATP hydrolysis rate is due to phosphorylation by PKA and the minor contribution (≤10%) to CFTR-ATPase activity. (ii) The mutant CFTR-E1371S that is still conductive, but defective in ATP hydrolysis, is not phosphorylated, suggesting that phosphorylation requires a functional nucleotide binding domain and occurs in the post-hydrolysis transition state. (iii) CFTR-ATPase activity is inversely related to CFTR anion flux. The present data are consistent with a model in which CFTR is in a closed conformation with two ATPs bound. The open conformation is induced by ATP hydrolysis and corresponds to the post-hydrolysis transition state that is stabilized by phosphorylation and binding of chloride channel potentiators.
Keywords: ABC transporter; biosensor; cyclic AMP (cAMP); cystic fibrosis transmembrane conductance regulator (CFTR); protein kinase A (PKA).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Riordan J. R. (2008) CFTR function and prospects for therapy. Annu. Rev. Biochem. 77, 701–726 - PubMed
-
- Qin L., Zheng J., Grant C. E., Jia Z., Cole S. P., and Deeley R. G. (2008) Residues responsible for the asymmetric function of the nucleotide binding domains of multidrug resistance protein 1. Biochemistry 47, 13952–13965 - PubMed
-
- Aleksandrov A. A., and Riordan J. R. (1998) Regulation of CFTR ion channel gating by MgATP. FEBS Lett. 431, 97–101 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

