Insights into Lysine Deacetylation of Natively Folded Substrate Proteins by Sirtuins
- PMID: 27226597
- PMCID: PMC4938187
- DOI: 10.1074/jbc.M116.726307
Insights into Lysine Deacetylation of Natively Folded Substrate Proteins by Sirtuins
Abstract
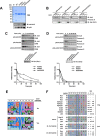
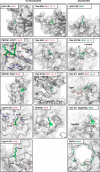
Sirtuins are NAD(+)-dependent lysine deacylases, regulating a variety of cellular processes. The nuclear Sirt1, the cytosolic Sirt2, and the mitochondrial Sirt3 are robust deacetylases, whereas the other sirtuins have preferences for longer acyl chains. Most previous studies investigated sirtuin-catalyzed deacylation on peptide substrates only. We used the genetic code expansion concept to produce natively folded, site-specific, and lysine-acetylated Sirt1-3 substrate proteins, namely Ras-related nuclear, p53, PEPCK1, superoxide dismutase, cyclophilin D, and Hsp10, and analyzed the deacetylation reaction. Some acetylated proteins such as Ras-related nuclear, p53, and Hsp10 were robustly deacetylated by Sirt1-3. However, other reported sirtuin substrate proteins such as cyclophilin D, superoxide dismutase, and PEPCK1 were not deacetylated. Using a structural and functional approach, we describe the ability of Sirt1-3 to deacetylate two adjacent acetylated lysine residues. The dynamics of this process have implications for the lifetime of acetyl modifications on di-lysine acetylation sites and thus constitute a new mechanism for the regulation of proteins by acetylation. Our studies support that, besides the primary sequence context, the protein structure is a major determinant of sirtuin substrate specificity.
Keywords: GTPase; acetylation; p53; sirtuin; synthetic biology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Choudhary C., Kumar C., Gnad F., Nielsen M. L., Rehman M., Walther T. C., Olsen J. V., and Mann M. (2009) Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 - PubMed
-
- Choudhary C., Weinert B. T., Nishida Y., Verdin E., and Mann M. (2014) The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

