A Common Signal Patch Drives AP-1 Protein-dependent Golgi Export of Inwardly Rectifying Potassium Channels
- PMID: 27226616
- PMCID: PMC4946915
- DOI: 10.1074/jbc.M116.729822
A Common Signal Patch Drives AP-1 Protein-dependent Golgi Export of Inwardly Rectifying Potassium Channels
Abstract
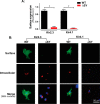
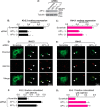
Nearly all members of the inwardly rectifying potassium (Kir) channel family share a cytoplasmic domain structure that serves as an unusual AP-1 clathrin adaptor-dependent Golgi export signal in one Kir channel, Kir2.1 (KCNJ2), raising the question whether Kir channels share a common Golgi export mechanism. Here we explore this idea, focusing on two structurally and functionally divergent Kir family members, Kir2.3 (KCNJ4) and Kir4.1/5.1 (KCNJ10/16), which have ∼50% amino identity. We found that Golgi export of both channels is blocked upon siRNA-mediated knockdown of the AP-1 γ subunit, as predicted for the common AP-1-dependent trafficking process. A comprehensive mutagenic analysis, guided by homology mapping in atomic resolution models of Kir2.1, Kir2.3, and Kir4.1/5.1, identified a common structure that serves as a recognition site for AP-1 binding and governs Golgi export. Larger than realized from previous studies with Kir2.1, the signal is created by a patch of residues distributed at the confluence of cytoplasmic N and C termini. The signal involves a stretch of hydrophobic residues from the C-terminal region that form a hydrophobic cleft, an adjacent cluster of basic residues within the N terminus, and a potential network of salt bridges that join the N- and C-terminal poles together. Because patch formation and AP-1 binding are dependent on proper folding of the cytoplasmic domains, the signal provides a common quality control mechanism at the Golgi for Kir channels. These findings identify a new proteostatic mechanism that couples protein folding of channels to forward trafficking in the secretory pathway.
Keywords: adaptor protein; clathrin; intracellular trafficking; potassium channel; protein trafficking (Golgi).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Traub L. M., and Kornfeld S. (1997) The trans-Golgi network: a late secretory sorting station. Curr. Opin. Cell Biol. 9, 527–533 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

