A Rare Case of Transfusion Transmission of Hepatitis A Virus to Two Patients with Haematological Disease
- PMID: 27226795
- PMCID: PMC4872048
- DOI: 10.1159/000441910
A Rare Case of Transfusion Transmission of Hepatitis A Virus to Two Patients with Haematological Disease
Abstract
Background: This paper describes the transmission of hepatitis A virus (HAV) to two blood recipients from a healthy donor that later presented to the blood bank with jaundice.
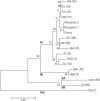
Methods: The RNA of HAV was detected by qualitative nested reverse transcription polymerase chain reaction (nested RT-PCR) and quantified by real-time RT-PCR. HAV RNA samples were genotyped by direct sequencing of PCR products. A sequence from a fragment of 168 bp from the VP1/2A HAV region was used to construct a phylogenetic tree.
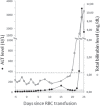
Case report: A 31-year-old male donor accepted for donation of a whole blood unit returned to the blood bank with clinical jaundice 20 days after donation. His serological and NAT tests were negative for HBV and HCV. Serological tests for HAV IgM and IgG were negative on donation sample but positive on follow-up sample, confirming donor's HAV acute infection. Both recipients of red blood cells (R1) and platelet concentrate (R2) from the same implicated donation were HAV IgM-negative and IgG-positive. Qualitative PCR was positive on samples from all three individuals and phylogenetic analysis of viruses proved HAV transmission to the two recipients of blood products. HAV viral load on donor follow-up sample and the platelet recipient was 1.3 and 1.5 × 10(3) IU/ml, respectively. The RBC recipient, also infected by HCV, was undergoing bone marrow transplantation and died from fulminant hepatitis, 26 days after the implicated HAV transfusion.
Conclusion: The blood donor, a garbage collector, spontaneously returned to the blood bank when developing jaundice. This highlights the importance of donor education to immediately report to blood banks of any signs and symptoms related to infectious disease developed after blood donation. The fact that one immunocompromised patient with HCV infection died from fulminant hepatitis after receiving a HAV-contaminated platelet transfusion underpins the importance of a HAV vaccination program for these group of patients.
Keywords: Blood donor; Blood transfusion; Hepatitis A; Immunosuppression; Window period.
Figures

References
-
- World Health Organization Hepatitis A. Fact sheet N°328 Updated July 2015. www.who.int/mediacentre/factsheets/fs328/en/ (last accessed October 26, 2015).
-
- Ximenes RA, Martelli CM, Amaku M, Sartori AM, de Soárez PC, Novaes HM, Pereira LM, Moreira RC, Figueiredo GM, de Azevedo RS, Hepatitis Study Group Modeling the force of infection for hepatitis A in an urban population-based survey: a comparison of transmission patterns in Brazilian macro-regions. PLoS One. 2014;20(9):e94622. - PMC - PubMed
-
- Yokosuka O. Molecular biology of hepatitis A virus: significance of various substitutions in the hepatitis A virus genome. J Gastroenterol Hepatol. 2000;15(suppl):D91–97. - PubMed
-
- Pereira E, Gonçalves C. Hepatitis A. Rev Soc Bras Med Trop. 2003;36:387–400. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

