Drug reformulations and repositioning in pharmaceutical industry and its impact on market access: reassessment of nomenclature
- PMID: 27226826
- PMCID: PMC4865745
- DOI: 10.3402/jmahp.v1i0.21131
Drug reformulations and repositioning in pharmaceutical industry and its impact on market access: reassessment of nomenclature
Abstract
Background: Medicinal products that have been developed and approved for one disease may be the object of additional clinical development in other disease areas or of additional pharmaceutical development for new and different formulations. The newly developed products can be named as repositioned or reformulated products, respectively. Market access of repositioned or reformulated products in Europe and the United States is an interesting object of study as it may provide clarity about which parameters are assessed and considered to bring added value, other than the molecule itself. As such, we aim to evaluate if the added value of repositioned or reformulated medicinal products can be systematically described, quantified, and predicted. As a first step toward investigating the impact of market access on drug research and development trends for repositioned and reformulated products, it is necessary to have consistency in the designations for the case studies evaluated in this project. In an attempt to achieve that consistency, the current study aims to propose harmonized definitions for the repositioning and reformulation strategies and to propose a taxonomy for the medicinal products derived thereof.
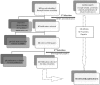
Methods: A systematic literature review was conducted to collect information on existing cases of repositioning or reformulation. A search strategy was developed by defining the search objectives, targeted data sources, search keywords, and inclusion/exclusion criteria for the retrieved documents.
Results: A total of 505 publications were retrieved through a search of the main data sources. The screenings and the ad hoc search led to a total of 56 publications to be used for the case study data extraction. In total, 87 repositioning and/or reformulation cases were found described in the literature, 23 of which presented different definitions and/or classifications by different authors.
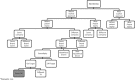
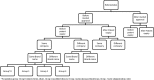
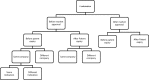
Conclusion: Given the disparity and inconsistency of terminologies and classifications in the literature, a harmonized nomenclature for drug repositioning, reformulation, and combination cases will allow for a robust analysis of the added value and market access conditions attributed for each strategy and case type as assessed by regulators and payors in Europe and the United States. After evaluation of the existing terminologies and given the absence of clear and consistent definitions for drug reformulation and repositioning in the literature, we propose a global terminology and taxonomy in order to cover all of the previously unclear definitions and classifications for repositioned and reformulated products.
Keywords: classification; combination; lifecycle management; market access; reformulation; repositioning; repurposing; taxonomy.
Figures


References
-
- Lekka E, Deftereos SN, Persidis A, Persidis A, Andronis C. Literature analysis for systematic drug repurposing: a case study from Biovista. Drug Discov Today Ther Strateg. 2012;8:103–8.
-
- Persidis A. The benefits of drug repositioning. Drug Discov World. 2011;12:9–12.
-
- Sleigh SH, Barton CL. Repurposing strategies for therapeutics. Pharm Med. 2010;24:151–9.
-
- Smith RB. Repositioned drugs: integrating intellectual property and regulatory strategies. Drug Discov Today Ther Strateg. 2011;(8):131–7.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
