Antifibrotic therapies to control cardiac fibrosis
- PMID: 27226899
- PMCID: PMC4879750
- DOI: 10.1186/s40824-016-0060-8
Antifibrotic therapies to control cardiac fibrosis
Abstract
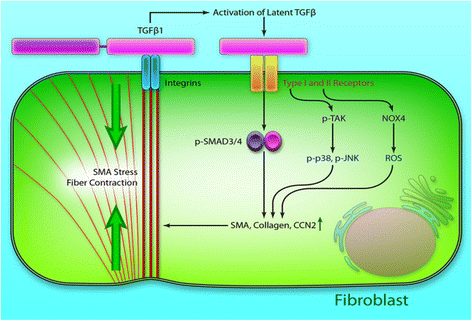
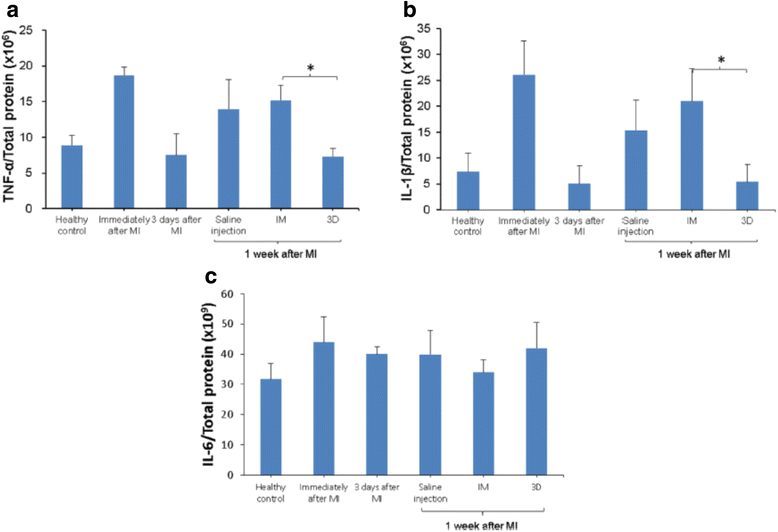
Cardiac fibrosis occurs naturally after myocardial infarction. While the initially formed fibrotic tissue prevents the infarcted heart tissue from rupture, the progression of cardiac fibrosis continuously expands the size of fibrotic tissue and causes cardiac function decrease. Cardiac fibrosis eventually evolves the infarcted hearts into heart failure. Inhibiting cardiac fibrosis from progressing is critical to prevent heart failure. However, there is no efficient therapeutic approach currently available. Myofibroblasts are primarily responsible for cardiac fibrosis. They are formed by cardiac fibroblast differentiation, fibrocyte differentiation, epithelial to mesenchymal transdifferentiation, and endothelial to mesenchymal transition, driven by cytokines such as transforming growth factor beta (TGF-β), angiotensin II and platelet-derived growth factor (PDGF). The approaches that inhibit myofibroblast formation have been demonstrated to prevent cardiac fibrosis, including systemic delivery of antifibrotic drugs, localized delivery of biomaterials, localized delivery of biomaterials and antifibrotic drugs, and localized delivery of cells using biomaterials. This review addresses current progresses in cardiac fibrosis therapies.
Keywords: Antifibrotic therapy; Cardiac fibroblasts; Cardiac fibrosis; Myocardial infarction; Myofibroblasts.
Figures



References
-
- Yarbrough WM, Mukherjee R, Stroud RE, Rivers WT, Oelsen JM, Dixon JA, et al. Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. J Thorac Cardiovasc Surg. 2012;143:215–23. doi: 10.1016/j.jtcvs.2011.09.032. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

