How genetically engineered systems are helping to define, and in some cases redefine, the neurobiological basis of sleep and wake
- PMID: 27227054
- PMCID: PMC4843941
- DOI: 10.1080/23328940.2015.1075095
How genetically engineered systems are helping to define, and in some cases redefine, the neurobiological basis of sleep and wake
Abstract
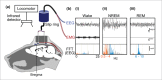
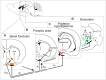
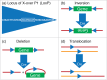
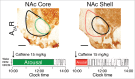
The advent of genetically engineered systems, including transgenic animals and recombinant viral vectors, has facilitated a more detailed understanding of the molecular and cellular substrates regulating brain function. In this review we highlight some of the most recent molecular biology and genetic technologies in the experimental "systems neurosciences," many of which are rapidly becoming a methodological standard, and focus in particular on those tools and techniques that permit the reversible and cell-type specific manipulation of neurons in behaving animals. These newer techniques encompass a wide range of approaches including conditional deletion of genes based on Cre/loxP technology, gene silencing using RNA interference, cell-type specific mapping or ablation and reversible manipulation (silencing and activation) of neurons in vivo. Combining these approaches with viral vector delivery systems, in particular adeno-associated viruses (AAV), has extended, in some instances greatly, the utility of these tools. For example, the spatially- and/or temporally-restricted transduction of specific neuronal cell populations is now routinely achieved using the combination of Cre-driver mice and stereotaxic-based delivery of AAV expressing Cre-dependent cassettes. We predict that the experimental application of these tools, including creative combinatorial approaches and the development of even newer reagents, will prove necessary for a complete understanding of the neuronal circuits subserving most neurobiological functions, including the regulation of sleep and wake.
Keywords: DREADD; RNA interference; adeno-associated virus; optogenetics; sleep-wake regulation.
Figures


Similar articles
-
tTARGIT AAVs mediate the sensitive and flexible manipulation of intersectional neuronal populations in mice.Elife. 2021 Mar 11;10:e66835. doi: 10.7554/eLife.66835. Elife. 2021. PMID: 33704065 Free PMC article.
-
Cre Activated and Inactivated Recombinant Adeno-Associated Viral Vectors for Neuronal Anatomical Tracing or Activity Manipulation.Curr Protoc Neurosci. 2015 Jul 1;72:1.24.1-1.24.15. doi: 10.1002/0471142301.ns0124s72. Curr Protoc Neurosci. 2015. PMID: 26131660 Free PMC article. Review.
-
Genetic Activation, Inactivation, and Deletion Reveal a Limited And Nuanced Role for Somatostatin-Containing Basal Forebrain Neurons in Behavioral State Control.J Neurosci. 2018 May 30;38(22):5168-5181. doi: 10.1523/JNEUROSCI.2955-17.2018. Epub 2018 May 7. J Neurosci. 2018. PMID: 29735555 Free PMC article.
-
Adeno-Associated Virus Technologies and Methods for Targeted Neuronal Manipulation.Front Neuroanat. 2019 Nov 26;13:93. doi: 10.3389/fnana.2019.00093. eCollection 2019. Front Neuroanat. 2019. PMID: 31849618 Free PMC article. Review.
-
Transgene expression in target-defined neuron populations mediated by retrograde infection with adeno-associated viral vectors.J Neurosci. 2013 Sep 18;33(38):15195-206. doi: 10.1523/JNEUROSCI.1618-13.2013. J Neurosci. 2013. PMID: 24048849 Free PMC article.
Cited by
-
The neuroanatomy and neurochemistry of sleep-wake control.Curr Opin Physiol. 2020 Jun;15:143-151. doi: 10.1016/j.cophys.2019.12.012. Epub 2019 Dec 31. Curr Opin Physiol. 2020. PMID: 32647777 Free PMC article.
-
Newly identified sleep-wake and circadian circuits as potential therapeutic targets.Sleep. 2019 May 1;42(5):zsz023. doi: 10.1093/sleep/zsz023. Sleep. 2019. PMID: 30722061 Free PMC article. Review.
-
Neural Circuitry Underlying Waking Up to Hypercapnia.Front Neurosci. 2019 Apr 26;13:401. doi: 10.3389/fnins.2019.00401. eCollection 2019. Front Neurosci. 2019. PMID: 31080401 Free PMC article. Review.
-
Validation of DREADD agonists and administration route in a murine model of sleep enhancement.J Neurosci Methods. 2022 Oct 1;380:109679. doi: 10.1016/j.jneumeth.2022.109679. Epub 2022 Jul 30. J Neurosci Methods. 2022. PMID: 35914577 Free PMC article.
-
Functionally Complete Excision of Conditional Alleles in the Mouse Suprachiasmatic Nucleus by Vgat-ires-Cre.J Biol Rhythms. 2018 Apr;33(2):179-191. doi: 10.1177/0748730418757006. J Biol Rhythms. 2018. PMID: 29671710 Free PMC article.
References
-
- Kohtoh S, Taguchi Y, Matsumoto N, Wada M, Huang Z-L, Urade Y. Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol Rhythms 2008; 6:163-71; http://dx.doi.org/10.1111/j.1479-8425.2008.00355.x - DOI
-
- Rosenbaum E. Warum müssen wir schlafen? : eine neue Theorie des Schlafes. Berlin: August Hirschwald, 1892.
-
- Kubota K. Kuniomi Ishimori and the first discovery of sleep-inducing substances in the brain. Neurosci Res 1989; 6:497-518; PMID:2677843; http://dx.doi.org/10.1016/0168-0102(89)90041-2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
