Direct-to-Patient Research: Piloting a New Approach to Understanding Drug Safety During Pregnancy
- PMID: 27227140
- PMCID: PMC4869223
- DOI: 10.2196/publichealth.4939
Direct-to-Patient Research: Piloting a New Approach to Understanding Drug Safety During Pregnancy
Abstract
Background: Little is known about the effects of human fetal exposure when a new drug is authorized unless it was specifically developed for use in pregnancy. Since many factors may contribute to adverse fetal effects, having comprehensive information about in utero exposures will enhance our ability to make correct determinations about causality.
Objective: The objective of the study was to assess the extent to which women, recruited without the intervention of health care professionals (HCPs), will provide information, suitable for research purposes, via the Internet or by phone on some potential risk factors in pregnancy.
Methods: To pilot direct-to-patient research for pharmacovigilance, we conducted a prospective, noninterventional study of medication use and lifestyle factors as part of the Pharmacoepidemiological Research on Outcomes of Therapeutics by a European ConsorTium (PROTECT) Consortium. Consenting women who self-identified as pregnant and residing in the United Kingdom (UK), Denmark (DK), The Netherlands, or Poland were recruited and could then choose to provide data every 2 or 4 weeks via the Internet or a telephonic interactive voice response system (IVRS). Self-reported drug use was compared with pharmacy register data in DK and with electronic health records in the UK.
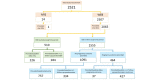
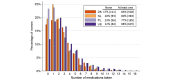
Results: Recruited women were on average older and more highly educated than the general population. Most respondents chose a frequency of every 4 weeks (56.99%, 1177/2065). Only 29.83% (464/1555) of women with due dates occurring during the study provided information on pregnancy outcome. For those responding by Internet, over 90.00% (1915/2065) reported using >1 pregnancy-related medication, 83.34% (1721/2065) reported using >1 other medicine, and 23.53% (486/2065) reported only over-the-counter medications, not counting herbals and dietary supplements. Some respondents (7.16%, 148/2065) reported that they chose not to take a prescribed medication (mostly medicines for pain or inflammation, and for depression) and 1.30% (27/2065) reported using medicines that had been prescribed to a friend or family member (oxycodone, paracetamol, and medications for acid-related problems). Relatively few respondents reported using fish oil (4.60%, 95/2065), other dietary supplements (1.88%, 39/2065), herbal products (7.07%, 146/2065), or homeopathic products (1.16%, 24/2065). Most medications for chronic conditions that were listed in the Danish prescription registry were also self-reported (83.3%, 145/174 agreement), with larger discrepancies for medications indicated for short-term use (54.0%, 153/283 agreement) and pregnancy-related medications (66.1%, 78/118).
Conclusions: Self-reported information on medication use as well as other potential teratogenic factors can be collected via the Internet, although recruitment costs are not insubstantial and maintaining follow-up is challenging. Direct data collection from consumers adds detail, but clinical input may be needed to fully understand patients' medical histories and capture birth outcomes.
Keywords: direct-to-patient; drug safety; pharmacovigilance; validation.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Brody Tm, Larner J, Minneman Kp. Human pharmacology: Molecular to clinical. St. Louis: Mosby; 1998. Perinatal/neonatal pharmacology.
LinkOut - more resources
Full Text Sources
Other Literature Sources

