Pericytes of the neurovascular unit: key functions and signaling pathways
- PMID: 27227366
- PMCID: PMC5745011
- DOI: 10.1038/nn.4288
Pericytes of the neurovascular unit: key functions and signaling pathways
Abstract
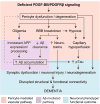
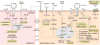
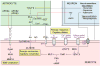
Pericytes are vascular mural cells embedded in the basement membrane of blood microvessels. They extend their processes along capillaries, pre-capillary arterioles and post-capillary venules. CNS pericytes are uniquely positioned in the neurovascular unit between endothelial cells, astrocytes and neurons. They integrate, coordinate and process signals from their neighboring cells to generate diverse functional responses that are critical for CNS functions in health and disease, including regulation of the blood-brain barrier permeability, angiogenesis, clearance of toxic metabolites, capillary hemodynamic responses, neuroinflammation and stem cell activity. Here we examine the key signaling pathways between pericytes and their neighboring endothelial cells, astrocytes and neurons that control neurovascular functions. We also review the role of pericytes in CNS disorders including rare monogenic diseases and complex neurological disorders such as Alzheimer's disease and brain tumors. Finally, we discuss directions for future studies.
Conflict of interest statement
Figures



References
-
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

