A Neuronal Culture System to Detect Prion Synaptotoxicity
- PMID: 27227882
- PMCID: PMC4881977
- DOI: 10.1371/journal.ppat.1005623
A Neuronal Culture System to Detect Prion Synaptotoxicity
Abstract
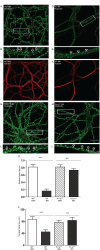
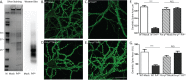
Synaptic pathology is an early feature of prion as well as other neurodegenerative diseases. Although the self-templating process by which prions propagate is well established, the mechanisms by which prions cause synaptotoxicity are poorly understood, due largely to the absence of experimentally tractable cell culture models. Here, we report that exposure of cultured hippocampal neurons to PrPSc, the infectious isoform of the prion protein, results in rapid retraction of dendritic spines. This effect is entirely dependent on expression of the cellular prion protein, PrPC, by target neurons, and on the presence of a nine-amino acid, polybasic region at the N-terminus of the PrPC molecule. Both protease-resistant and protease-sensitive forms of PrPSc cause dendritic loss. This system provides new insights into the mechanisms responsible for prion neurotoxicity, and it provides a platform for characterizing different pathogenic forms of PrPSc and testing potential therapeutic agents.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

