The Loss of Lam2 and Npr2-Npr3 Diminishes the Vacuolar Localization of Gtr1-Gtr2 and Disinhibits TORC1 Activity in Fission Yeast
- PMID: 27227887
- PMCID: PMC4881991
- DOI: 10.1371/journal.pone.0156239
The Loss of Lam2 and Npr2-Npr3 Diminishes the Vacuolar Localization of Gtr1-Gtr2 and Disinhibits TORC1 Activity in Fission Yeast
Abstract
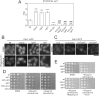
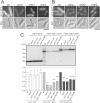
In mammalian cells, mTORC1 activity is regulated by Rag GTPases. It is thought that the Ragulator complex and the GATOR (GAP activity towards Rags) complex regulate RagA/B as its GDP/GTP exchange factor (GEF) and GTPase-activating protein (GAP), respectively. However, the functions of components in these complexes remain elusive. Using fission yeast as a model organism, here we found that the loss of Lam2 (SPBC1778.05c), a homolog of a Ragulator component LAMTOR2, as well as the loss of Gtr1 or Gtr2 phenocopies the loss of Npr2 or Npr3, homologs of GATOR components Nprl2 or Nprl3, respectively. These phenotypes were rescued by TORC1 inhibition using pharmacological or genetic means, and the loss of Lam2, Gtr1, Gtr2, Npr2 or Npr3 disinhibited TORC1 activity under nitrogen depletion, as measured by Rps6 phosphorylation. Consistently, overexpression of GDP-locked Gtr1S20L or GTP-locked Gtr2Q60L, which suppress TORC1 activity in budding yeast, rescued the growth defect of Δgtr1 cells or Δgtr2 cells, respectively, and the loss of Lam2, Npr2 or Npr3 similarly diminished the vacuolar localization and the protein levels of Gtr1 and Gtr2. Furthermore, Lam2 physically interacted with Npr2 and Gtr1. These findings suggest that Lam2 and Npr2-Npr3 function together as a tether for GDP-bound Gtr1 to the vacuolar membrane, thereby suppressing TORC1 activity for multiple cellular functions.
Conflict of interest statement
Figures







Similar articles
-
Reciprocal conversion of Gtr1 and Gtr2 nucleotide-binding states by Npr2-Npr3 inactivates TORC1 and induces autophagy.Autophagy. 2014 Sep;10(9):1565-78. doi: 10.4161/auto.29397. Epub 2014 Jun 30. Autophagy. 2014. PMID: 25046117 Free PMC article.
-
Dynamic relocation of the TORC1-Gtr1/2-Ego1/2/3 complex is regulated by Gtr1 and Gtr2.Mol Biol Cell. 2016 Jan 15;27(2):382-96. doi: 10.1091/mbc.E15-07-0470. Epub 2015 Nov 25. Mol Biol Cell. 2016. PMID: 26609069 Free PMC article.
-
The Vam6 and Gtr1-Gtr2 pathway activates TORC1 in response to amino acids in fission yeast.J Cell Sci. 2012 Apr 15;125(Pt 8):1920-8. doi: 10.1242/jcs.094219. Epub 2012 Feb 17. J Cell Sci. 2012. PMID: 22344254 Free PMC article.
-
TORC1-Dependent Phosphorylation Targets in Fission Yeast.Biomolecules. 2017 Jul 3;7(3):50. doi: 10.3390/biom7030050. Biomolecules. 2017. PMID: 28671615 Free PMC article. Review.
-
Target of rapamycin signaling mediates vacuolar fragmentation.Curr Genet. 2017 Feb;63(1):35-42. doi: 10.1007/s00294-016-0616-0. Epub 2016 May 27. Curr Genet. 2017. PMID: 27233284 Free PMC article. Review.
Cited by
-
Conserved and Divergent Mechanisms That Control TORC1 in Yeasts and Mammals.Genes (Basel). 2021 Jan 12;12(1):88. doi: 10.3390/genes12010088. Genes (Basel). 2021. PMID: 33445779 Free PMC article. Review.
-
Effect of Amino Acids on Fusarium oxysporum Growth and Pathogenicity Regulated by TORC1-Tap42 Gene and Related Interaction Protein Analysis.Foods. 2023 Apr 28;12(9):1829. doi: 10.3390/foods12091829. Foods. 2023. PMID: 37174368 Free PMC article.
-
Ragulator and GATOR1 complexes promote fission yeast growth by attenuating TOR complex 1 through Rag GTPases.Elife. 2017 Dec 4;6:e30880. doi: 10.7554/eLife.30880. Elife. 2017. PMID: 29199950 Free PMC article.
-
Novel Links between TORC1 and Traditional Non-Coding RNA, tRNA.Genes (Basel). 2020 Aug 19;11(9):956. doi: 10.3390/genes11090956. Genes (Basel). 2020. PMID: 32825021 Free PMC article. Review.
-
Effect of astragalosides on long non-coding RNA expression profiles in rats with adjuvant-induced arthritis.Int J Mol Med. 2019 Oct;44(4):1344-1356. doi: 10.3892/ijmm.2019.4281. Epub 2019 Jul 22. Int J Mol Med. 2019. PMID: 31364738 Free PMC article.
References
-
- Hayashi T, Hatanaka M, Nagao K, Nakaseko Y, Kanoh J, Kokubu A, et al. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes to cells: devoted to molecular & cellular mechanisms. 2007;12(12):1357–70. 10.1111/j.1365-2443.2007.01141.x . - DOI - PubMed
-
- Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. The Journal of biological chemistry. 2005;280(19):18717–27. 10.1074/jbc.M414499200 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

