Na+ Influx Induced by New Antimalarials Causes Rapid Alterations in the Cholesterol Content and Morphology of Plasmodium falciparum
- PMID: 27227970
- PMCID: PMC4881962
- DOI: 10.1371/journal.ppat.1005647
Na+ Influx Induced by New Antimalarials Causes Rapid Alterations in the Cholesterol Content and Morphology of Plasmodium falciparum
Abstract
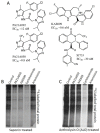
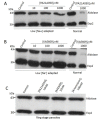
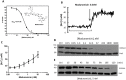
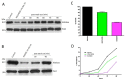
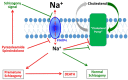
Among the several new antimalarials discovered over the past decade are at least three clinical candidate drugs, each with a distinct chemical structure, that disrupt Na+ homeostasis resulting in a rapid increase in intracellular Na+ concentration ([Na+]i) within the erythrocytic stages of Plasmodium falciparum. At present, events triggered by Na+ influx that result in parasite demise are not well-understood. Here we report effects of two such drugs, a pyrazoleamide and a spiroindolone, on intraerythrocytic P. falciparum. Within minutes following the exposure to these drugs, the trophozoite stage parasite, which normally contains little cholesterol, was made permeant by cholesterol-dependent detergents, suggesting it acquired a substantial amount of the lipid. Consistently, the merozoite surface protein 1 and 2 (MSP1 and MSP2), glycosylphosphotidylinositol (GPI)-anchored proteins normally uniformly distributed in the parasite plasma membrane, coalesced into clusters. These alterations were not observed following drug treatment of P. falciparum parasites adapted to grow in a low [Na+] growth medium. Both cholesterol acquisition and MSP1 coalescence were reversible upon the removal of the drugs, implicating an active process of cholesterol exclusion from trophozoites that we hypothesize is inhibited by high [Na+]i. Electron microscopy of drug-treated trophozoites revealed substantial morphological changes normally seen at the later schizont stage including the appearance of partial inner membrane complexes, dense organelles that resemble "rhoptries" and apparent nuclear division. Together these results suggest that [Na+]i disruptor drugs by altering levels of cholesterol in the parasite, dysregulate trophozoite to schizont development and cause parasite demise.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Dramatic Consequences of Reducing Erythrocyte Membrane Cholesterol on Plasmodium falciparum.Microbiol Spectr. 2022 Feb 23;10(1):e0015822. doi: 10.1128/spectrum.00158-22. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196803 Free PMC article.
-
Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum.Nat Commun. 2014 Nov 25;5:5521. doi: 10.1038/ncomms6521. Nat Commun. 2014. PMID: 25422853 Free PMC article.
-
Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials.Cell Host Microbe. 2013 Feb 13;13(2):227-37. doi: 10.1016/j.chom.2012.12.006. Cell Host Microbe. 2013. PMID: 23414762 Free PMC article.
-
The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs.Int J Parasitol Drugs Drug Resist. 2015 Aug 27;5(3):149-62. doi: 10.1016/j.ijpddr.2015.07.001. eCollection 2015 Dec. Int J Parasitol Drugs Drug Resist. 2015. PMID: 26401486 Free PMC article. Review.
-
Ion metabolism in malaria-infected erythrocytes.Blood Cells. 1990;16(2-3):437-49. Blood Cells. 1990. PMID: 2175223 Review.
Cited by
-
The Plasmodium falciparum NCR1 transporter is an antimalarial target that exports cholesterol from the parasite's plasma membrane.Sci Adv. 2024 Dec 20;10(51):eadq6651. doi: 10.1126/sciadv.adq6651. Epub 2024 Dec 18. Sci Adv. 2024. PMID: 39693420 Free PMC article.
-
Conditional permeabilization of the P. falciparum plasma membrane in infected cells links cation influx to reduced membrane integrity.PLoS One. 2023 Apr 4;18(4):e0283776. doi: 10.1371/journal.pone.0283776. eCollection 2023. PLoS One. 2023. PMID: 37014920 Free PMC article.
-
Diverse Chemical Compounds Target Plasmodium falciparum Plasma Membrane Lipid Homeostasis.ACS Infect Dis. 2019 Apr 12;5(4):550-558. doi: 10.1021/acsinfecdis.8b00277. Epub 2019 Jan 28. ACS Infect Dis. 2019. PMID: 30638365 Free PMC article.
-
Prefoldin subunit 6 of Plasmodium falciparum binds merozoite surface protein-1.FEBS Open Bio. 2022 May;12(5):1050-1060. doi: 10.1002/2211-5463.13022. Epub 2022 Mar 29. FEBS Open Bio. 2022. PMID: 33145997 Free PMC article.
-
Dramatic Consequences of Reducing Erythrocyte Membrane Cholesterol on Plasmodium falciparum.Microbiol Spectr. 2022 Feb 23;10(1):e0015822. doi: 10.1128/spectrum.00158-22. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196803 Free PMC article.
References
-
- Spillman NJ, Allen RJ, McNamara CW, Yeung BK, Winzeler EA, et al. (2013) Na(+) regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13: 227–237. 10.1016/j.chom.2012.12.006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

