'Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich's ataxia'
- PMID: 27228352
- PMCID: PMC4917650
- DOI: 10.1038/cddis.2016.111
'Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich's ataxia'
Abstract
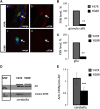
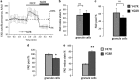
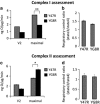
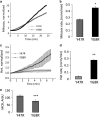
Friedreich's ataxia (FRDA) is an inherited neurodegenerative disease. The mutation consists of a GAA repeat expansion within the FXN gene, which downregulates frataxin, leading to abnormal mitochondrial iron accumulation, which may in turn cause changes in mitochondrial function. Although, many studies of FRDA patients and mouse models have been conducted in the past two decades, the role of frataxin in mitochondrial pathophysiology remains elusive. Are the mitochondrial abnormalities only a side effect of the increased accumulation of reactive iron, generating oxidative stress? Or does the progressive lack of iron-sulphur clusters (ISCs), induced by reduced frataxin, cause an inhibition of the electron transport chain complexes (CI, II and III) leading to reactive oxygen species escaping from oxidative phosphorylation reactions? To answer these crucial questions, we have characterised the mitochondrial pathophysiology of a group of disease-relevant and readily accessible neurons, cerebellar granule cells, from a validated FRDA mouse model. By using live cell imaging and biochemical techniques we were able to demonstrate that mitochondria are deregulated in neurons from the YG8R FRDA mouse model, causing a decrease in mitochondrial membrane potential (▵Ψm) due to an inhibition of Complex I, which is partially compensated by an overactivation of Complex II. This complex activity imbalance leads to ROS generation in both mitochondrial matrix and cytosol, which results in glutathione depletion and increased lipid peroxidation. Preventing this increase in lipid peroxidation, in neurons, protects against in cell death. This work describes the pathophysiological properties of the mitochondria in neurons from a FRDA mouse model and shows that lipid peroxidation could be an important target for novel therapeutic strategies in FRDA, which still lacks a cure.
Figures



References
-
- Harding AE. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 1981; 104: 589–620. - PubMed
-
- Parkinson MH, Boesch S, Nachbauer W, Mariotti C, Giunti P. Clinical features of Friedreich's ataxia: classical and atypical phenotypes. J Neurochem 2013; 126: 103–117. - PubMed
-
- Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F et al. Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996; 271: 1423–1427. - PubMed
-
- Pandolfo M, Pastore A. The pathogenesis of Friedreich ataxia and the structure and function of frataxin. J Neurol 2009; 256: 9–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

