Enhanced Inflammatory Transcriptome in the Granulosa Cells of Women With Polycystic Ovarian Syndrome
- PMID: 27228368
- PMCID: PMC5010574
- DOI: 10.1210/jc.2015-4275
Enhanced Inflammatory Transcriptome in the Granulosa Cells of Women With Polycystic Ovarian Syndrome
Abstract
Context: Polycystic ovarian syndrome (PCOS), the most common endocrine disorder of reproductive-aged women, is associated with systemic low-grade inflammation.
Objective: We propose that increased or altered intrafollicular inflammatory reactions also occur in periovulatory follicles of PCOS patients.
Design: Gene profiling and quantitative PCR (qPCR) analyses in granulosa-lutein cells (GCs) collected from PCOS and non-PCOS women undergoing in vitro fertilization were compared with serum and follicular fluid (FF) levels of cytokines and chemokines.
Setting: This was a university-based study.
Patients: Twenty-one PCOS and 45 control patients were recruited: demographic, hormone, body mass index, and pregnancy outcomes were abstracted from patient data files.
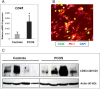
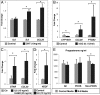
Interventions: GC cytokine/chemokine mRNAs were identified and analyzed by gene-chip microarrays/qPCR before and after culture with human chorionic gonadotropin, DHT, IL-6, or IL-8; serum/FF cytokine levels were also analyzed.
Main outcome measures: Relative serum/FF cytokine levels and GC cytokine expression before and after culture were compared and related to body mass index.
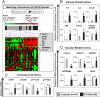
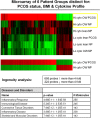
Results: The following results were found: 1) PCOS GCs express elevated transcripts encoding cytokines, chemokines, and immune cell markers, 2) based on gene profiling and qPCR analyses, obese PCOS patients define a distinct PCOS disease subtype with the most dramatic increases in proinflammatory and immune-related factors, and 3) human chorionic gonadotropin and DHT increased cytokine production in cultured GCs, whereas cytokines augmented cytokine and vascular genes, indicating that hyperandrogenism/elevated LH and obesity in PCOS women augment intrafollicular cytokine production.
Conclusions: Intrafollicular androgens and cytokines likely comprise a local regulatory loop that impacts GC expression of cytokines and chemokines and the presence of immune cells; this loop is further enhanced in the obese PCOS subtype.
Figures

References
-
- Norman RJ, Brannstrom M. Cytokines in the ovary: pathophysiology and potential for pharmacological intervention. Pharmacol Ther. 1996;69(3):219–236. - PubMed
-
- Hellberg P, Thomsen P, Janson PO, Brannstrom M. Leukocyte supplementation increases the luteinizing hormone-induced ovulation rate in the in vitro-perfused rat ovary. Biol Reprod. 1991;44(5):791–797. - PubMed
-
- Brannstrom M, Bonello N, Wang LJ, Norman RJ. Effects of tumour necrosis factor α (TNF α) on ovulation in the rat ovary. Reprod Fertil Dev. 1995;7(1):67–73. - PubMed
-
- Brannstrom M, Bonello N, Norman RJ, Robertson SA. Reduction of ovulation rate in the rat by administration of a neutrophil-depleting monoclonal antibody. J Reprod Immunol. 1995;29(3):265–270. - PubMed
-
- Smolikova K, Mlynarcikova A, Scsukova S. Role of interleukins in the regulation of ovarian functions. Endocr Regul. 2012;46(4):237–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

