A mathematical model for the simulation of the formation and the subsequent regression of hypertrophic scar tissue after dermal wounding
- PMID: 27229739
- PMCID: PMC5285433
- DOI: 10.1007/s10237-016-0799-9
A mathematical model for the simulation of the formation and the subsequent regression of hypertrophic scar tissue after dermal wounding
Abstract
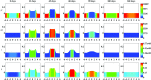
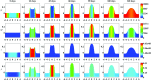
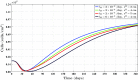
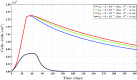
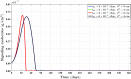
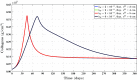
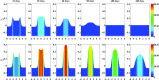
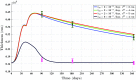
A continuum hypothesis-based model is presented for the simulation of the formation and the subsequent regression of hypertrophic scar tissue after dermal wounding. Solely the dermal layer of the skin is modeled explicitly and it is modeled as a heterogeneous, isotropic and compressible neo-Hookean solid. With respect to the constituents of the dermal layer, the following components are selected as primary model components: fibroblasts, myofibroblasts, a generic signaling molecule and collagen molecules. A good match with respect to the evolution of the thickness of the dermal layer of scars between the outcomes of simulations and clinical measurements on hypertrophic scars at different time points after injury in human subjects is demonstrated. Interestingly, the comparison between the outcomes of the simulations and the clinical measurements demonstrates that a relatively high apoptosis rate of myofibroblasts results in scar tissue that behaves more like normal scar tissue with respect to the evolution of the thickness of the tissue over time, while a relatively low apoptosis rate results in scar tissue that behaves like hypertrophic scar tissue with respect to the evolution of the thickness of the tissue over time. Our ultimate goal is to construct models with which the properties of newly generated tissues that form during wound healing can be predicted with a high degree of certainty. The development of the presented model is considered by us as a step toward their construction.
Keywords: Biomechanics; Compressible neo-Hookean solid; Dermal wound healing; Fibroblasts; Flux-corrected transport (FCT) limiter; Hypertrophic scar tissue; Modeling; Moving boundary; Moving-grid finite-element method.
Conflict of interest statement
Compliance with ethical standards Funding This study was funded by a grant from the Dutch Burns Foundation (project number WO/12.103). Conflict of interest The authors declare that they have no conflict of interest.
Figures

References
-
- Abe R, Donneley S, Peng T, Bucala R, Metz C. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:756–762. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

