Engineering galectin-glycan interactions for immunotherapy and immunomodulation
- PMID: 27229902
- PMCID: PMC4950369
- DOI: 10.1177/1535370216650055
Engineering galectin-glycan interactions for immunotherapy and immunomodulation
Abstract
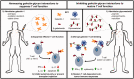
Galectins, a 15-member family of soluble carbohydrate-binding proteins, are receiving increasing interest as therapeutic targets for immunotherapy and immunomodulation due to their role as extracellular signals that regulate innate and adaptive immune cell phenotype and function. However, different galectins can have redundant, synergistic, or antagonistic signaling activity in normal immunological responses, such as resolution of inflammation and induction of antigen-specific tolerance. In addition, certain galectins can be hijacked to promote progression of immunopathologies, such as tumor immune privilege, metastasis, and viral infection, while others can inhibit these processes. Thus, eliciting a desired immunological outcome will likely necessitate therapeutics that can precisely enhance or inhibit particular galectin-glycan interactions. Multivalency is an important determinant of the affinity and specificity of natural galectin-glycan interactions, and is emerging as a key design element for therapeutics that can effectively manipulate galectin bioactivity. This minireview surveys current molecular and biomaterial engineering approaches to create therapeutics that can stabilize galectin multivalency or recapitulate natural glycan multivalency (i.e. "the glycocluster effect"). In particular, we highlight examples of using natural and engineered multivalent galectins for immunosuppression and immune tolerance, with a particular emphasis on treating autoimmune diseases or avoiding transplant rejection. In addition, we present examples of multivalent inhibitors of galectin-glycan interactions to maintain or restore T-cell function, with a particular emphasis on promoting antitumor immunity. Finally, we discuss emerging opportunities to further engineer galectin-glycan interactions for immunotherapy and immunomodulation.
Keywords: Biomaterial; bionanoscience; engineering; glycan; immunobiology; immunology.
© 2016 by the Society for Experimental Biology and Medicine.
Figures



References
-
- Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 2004; 14: 53R–62R. - PubMed
-
- Yamamoto K. Intracellular lectins involved in folding and transport in the endoplasmic reticulum. Biol Pharm Bull 2009; 32: 767–73. - PubMed
-
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science 2001; 291: 2364–9. - PubMed
-
- Kaltner H, Stierstorfer B. Animal lectins as cell adhesion molecules. Acta Anat (Basel) 1998; 161: 162–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

