Production of para-aminobenzoic acid from different carbon-sources in engineered Saccharomyces cerevisiae
- PMID: 27230236
- PMCID: PMC4882779
- DOI: 10.1186/s12934-016-0485-8
Production of para-aminobenzoic acid from different carbon-sources in engineered Saccharomyces cerevisiae
Abstract
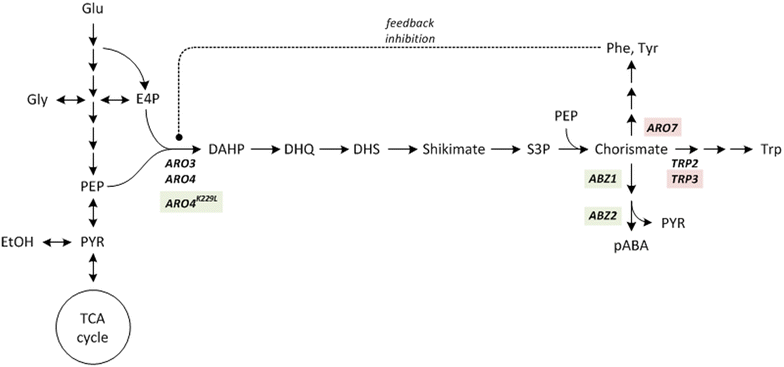
Background: Biological production of the aromatic compound para-aminobenzoic acid (pABA) is of great interest to the chemical industry. Besides its application in pharmacy and as crosslinking agent for resins and dyes pABA is a potential precursor for the high-volume aromatic feedstocks terephthalic acid and para-phenylenediamine. The yeast Saccharomyces cerevisiae synthesises pABA in the shikimate pathway: Outgoing from the central shikimate pathway intermediate chorismate, pABA is formed in two enzyme-catalysed steps, encoded by the genes ABZ1 and ABZ2. In this study S. cerevisiae metabolism was genetically engineered for the overproduction of pABA. Using in silico metabolic modelling an observed impact of carbon-source on product yield was investigated and exploited to optimize production.
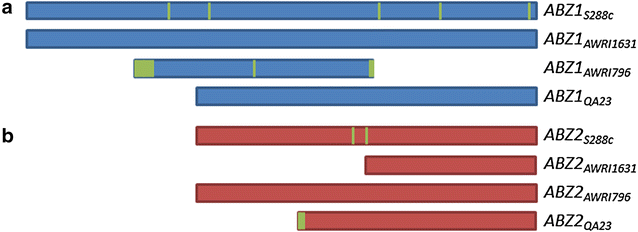
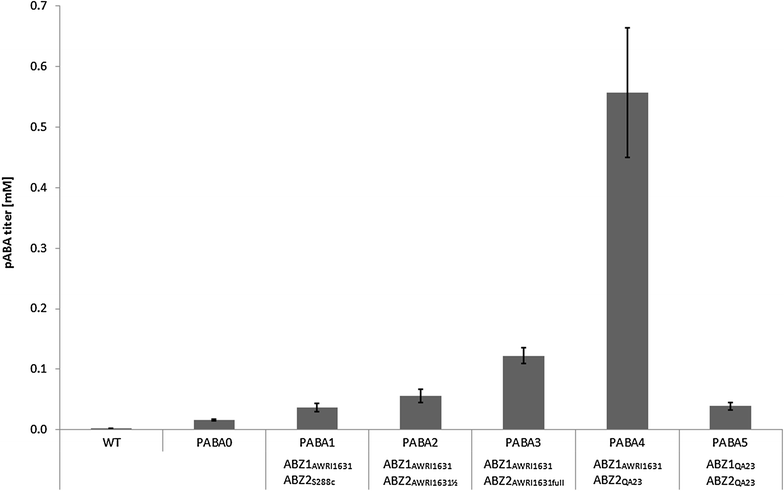
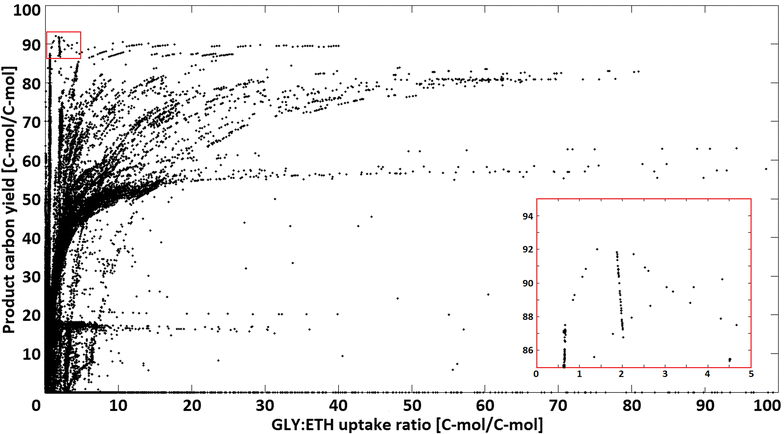
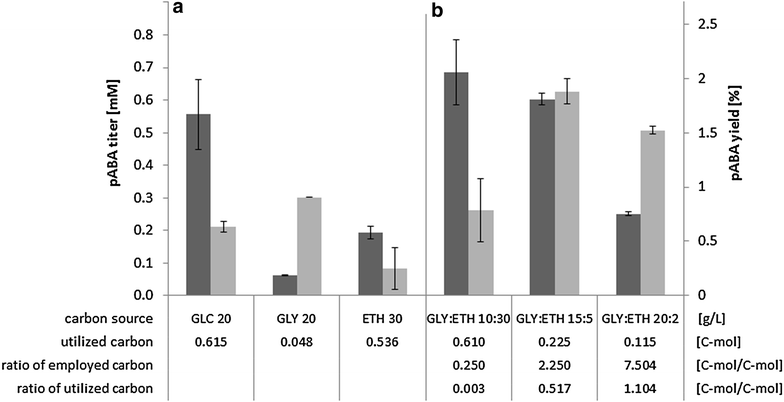
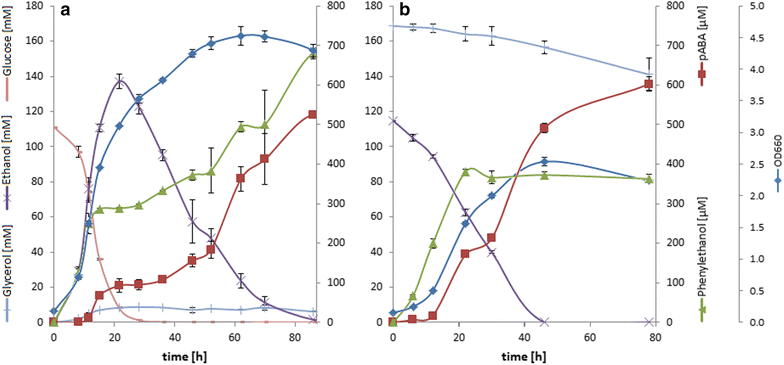
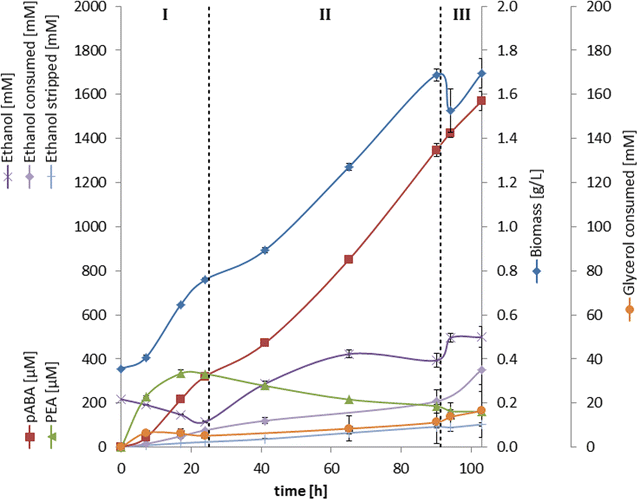
Results: A strain that incorporated the feedback resistant ARO4 (K229L) and deletions in the ARO7 and TRP3 genes, in order to channel flux to chorismate, was used to screen different ABZ1 and ABZ2 genes for pABA production. In glucose based shake-flaks fermentations the highest titer (600 µM) was reached when over-expressing the ABZ1 and ABZ2 genes from the wine yeast strains AWRI1631 and QA23, respectively. In silico metabolic modelling indicated a metabolic advantage for pABA production on glycerol and combined glycerol-ethanol carbon-sources. This was confirmed experimentally, the empirical ideal glycerol to ethanol uptake ratios of 1:2-2:1 correlated with the model. A (13)C tracer experiment determined that up to 32% of the produced pABA originated from glycerol. Finally, in fed-batch bioreactor experiments pABA titers of 1.57 mM (215 mg/L) and carbon yields of 2.64% could be achieved.
Conclusion: In this study a combination of genetic engineering and in silico modelling has proven to be a complete and advantageous approach to increase pABA production. Especially the enzymes that catalyse the last two steps towards product formation appeared to be crucial to direct flux to pABA. A stoichiometric model for carbon-utilization proved useful to design carbon-source composition, leading to increased pABA production. The reported pABA concentrations and yields are, to date, the highest in S. cerevisiae and the second highest in a microbial production system, underlining the great potential of yeast as a cell factory for renewable aromatic feedstocks.
Keywords: Aromatics; Ethanol; Glycerol; Phenylethanol; Yeast; pABA.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

