Evaluating the Survival Benefit Following Ovarian Function Suppression in Premenopausal Patients with Hormone Receptor Positive Early Breast Cancer
- PMID: 27230285
- PMCID: PMC4882507
- DOI: 10.1038/srep26627
Evaluating the Survival Benefit Following Ovarian Function Suppression in Premenopausal Patients with Hormone Receptor Positive Early Breast Cancer
Abstract
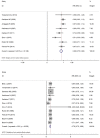
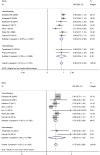
There are divergent opinions regarding the use of ovarian function suppression or ablation (hereafter, OFS) in hormone receptor positive early breast cancer patients. In order to clarify the survival benefit of OFS, a meta-analysis was performed. The result is that use of OFS was more effective than no OFS on DFS (the pooled relative risk (pRR) = 0.86; 95% CI: 0.75-0.96) and on OS (pRR = 0.79; 95% CI: 0.70-0.89). In subgroup analysis, we found that increased DFS was positively associated with patients who had received chemotherapy (pRR = 0.85; 95% CI: 0.74-0.96), who were lymph node negative (pRR = 0.74; 95% CI: 0.61-0.91) and were less than 40 years old (pRR = 0.71; 95% CI: 0.59-0.83). There was a significant difference in OS between the groups receiving chemotherapy (pRR = 0.73; 95% CI: 0.58-0.89) or for patients less than 40 years old (pRR = 0.52; 95% CI: 0.18-0.87). The use of OFS also produces statistical differences in the occurrence of the side-effects; severe hot flashes (pRR = 2.32; 95% CI: 1.36-3.97), and hypertension (pRR = 1.54; 95% CI: 1.12-2.12). In general, OFS should be considered as one treatment for hormone receptor positive premenopausal early breast cancer patients who have received chemotherapy and are less than 40 years old. We also should pay attention to the side-effects and weigh the advantages and disadvantages before deciding on using OFS.
Figures
References
-
- Buzdar A. U. & Hortobagyi G. Update on endocrine therapy for breast cancer. Clinical Cancer Research 4, 527–534 (1998). - PubMed
-
- Powles T. J. The case for clinical trials of tamoxifen for prevention of breast cancer. Lancet 340, 1145–1147 (1992). - PubMed
-
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet 351, 1451–1467 (1998). - PubMed
-
- Fisher B., Digman J., Bryant J., DeCillis A., Wickerham D. L. et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 88, 1529–1542 (1996). - PubMed
-
- Fisher B. et al. Tamoxifen for prevention of breast cancer: Report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst 90, 1371–1388 (1998). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical