Mouse chromosome 2 harbors genetic determinants of resistance to podocyte injury and renal tubulointerstitial fibrosis
- PMID: 27230548
- PMCID: PMC4882790
- DOI: 10.1186/s12863-016-0378-1
Mouse chromosome 2 harbors genetic determinants of resistance to podocyte injury and renal tubulointerstitial fibrosis
Abstract
Background: Tensin2 deficiency results in alterations in podocytes and subsequent glomerular and tubulointerstitial injuries. However, this pathology is critically dependent on genetic background. While the Tensin2-deficient podocytes of resistant murine strains, including C57BL/6J mice, remain almost intact, susceptible murine strains with Tensin2 deficency, including ICGN mice, develop chronic kidney disease following alterations in the podocyte foot processes. In a previous study, genome-wide linkage analysis was utilized to identify the quantitative trait loci associated with the disease phenotypes on mouse chromosome 2. This study investigated the disease phenotypes of chromosome 2 consomic and subcongenic strains.
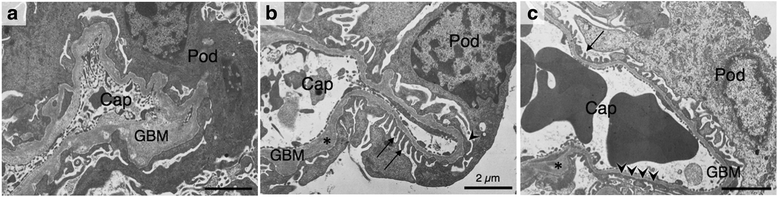
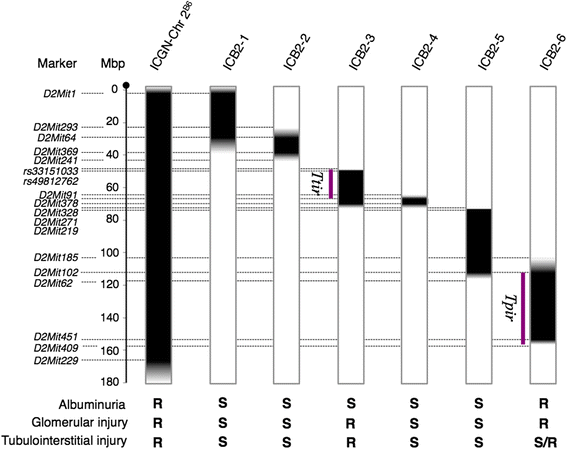
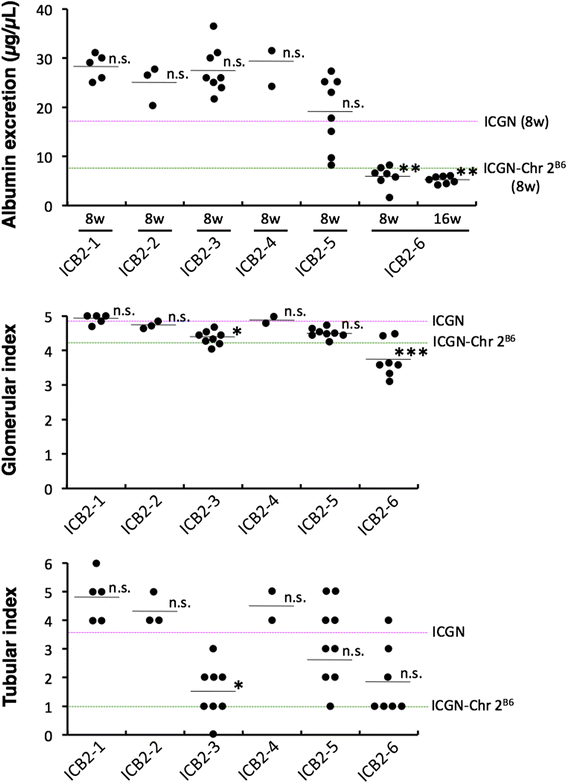
Results: ICGN consomic mice introgressed with chromosome 2 from the C57BL/6J mouse were generated and found to exhibit milder renal failure than that in ICGN mice. We developed 6 subcongenic strains that carry C57BL/6J-derived chromosomal segments from the consomic strain. One showed significantly milder albuminuria, another showed significantly milder tubulointerstitial injury, and the both showed significantly milder glomerular injury.
Conclusions: These data indicate that mouse chromosome 2 harbors two major genes associated with the severities of nephropathy induced by Tensin2 deficiency. The proximal region on chromosome 2 contributes to the resistance to tubulointerstitial fibrosis. In contrast, the distal region on chromosome 2 contributes to the resistance to podocyte injury. This study would be helpful to discover the biological mechanism underlying the renal injury, and may lead to the identification of therapeutic targets.
Keywords: Albuminuria; Chronic kidney disease; Renal fibrosis; Tensin2.
Figures


Similar articles
-
Genetic loci for resistance to podocyte injury caused by the tensin2 gene deficiency in mice.BMC Genet. 2018 Apr 10;19(1):24. doi: 10.1186/s12863-018-0611-1. BMC Genet. 2018. PMID: 29636014 Free PMC article.
-
Lysyl oxidase‑like 2 is expressed in kidney tissue and is associated with the progression of tubulointerstitial fibrosis.Mol Med Rep. 2017 Sep;16(3):2477-2482. doi: 10.3892/mmr.2017.6918. Epub 2017 Jul 4. Mol Med Rep. 2017. PMID: 28677767 Free PMC article.
-
Genetic background-dependent diversity in renal failure caused by the tensin2 gene deficiency in the mouse.Biomed Res. 2015;36(5):323-30. doi: 10.2220/biomedres.36.323. Biomed Res. 2015. PMID: 26522149
-
Complex quantitative traits cracked by the mouse inter-subspecific consomic strains.Exp Anim. 2012;61(4):375-88. doi: 10.1538/expanim.61.375. Exp Anim. 2012. PMID: 22850637 Review.
-
[The role of podocyte injury in chronic kidney disease].Nihon Rinsho Meneki Gakkai Kaishi. 2015;38(1):26-36. doi: 10.2177/jsci.38.26. Nihon Rinsho Meneki Gakkai Kaishi. 2015. PMID: 25765686 Review. Japanese.
Cited by
-
Novel murine model of congenital diabetes: The insulin hyposecretion mouse.J Diabetes Investig. 2019 Mar;10(2):227-237. doi: 10.1111/jdi.12895. Epub 2018 Aug 17. J Diabetes Investig. 2019. PMID: 29987871 Free PMC article.
-
Differences in susceptibility to ADR nephropathy among C57BL/6 substrains.Exp Anim. 2023 Nov 9;72(4):520-525. doi: 10.1538/expanim.23-0003. Epub 2023 Jun 21. Exp Anim. 2023. PMID: 37344407 Free PMC article.
-
Tensins in Kidney Function and Diseases.Life (Basel). 2023 May 24;13(6):1244. doi: 10.3390/life13061244. Life (Basel). 2023. PMID: 37374025 Free PMC article. Review.
-
Tensin 2-deficient nephropathy: mechanosensitive nephropathy, genetic susceptibility.Exp Anim. 2022 Aug 5;71(3):252-263. doi: 10.1538/expanim.22-0031. Epub 2022 Apr 19. Exp Anim. 2022. PMID: 35444113 Free PMC article. Review.
-
Genetic loci for resistance to podocyte injury caused by the tensin2 gene deficiency in mice.BMC Genet. 2018 Apr 10;19(1):24. doi: 10.1186/s12863-018-0611-1. BMC Genet. 2018. PMID: 29636014 Free PMC article.
References
-
- Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–259. doi: 10.1038/sj.ki.5002343. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

