Gaining and sustaining schistosomiasis control: study protocol and baseline data prior to different treatment strategies in five African countries
- PMID: 27230666
- PMCID: PMC4880878
- DOI: 10.1186/s12879-016-1575-2
Gaining and sustaining schistosomiasis control: study protocol and baseline data prior to different treatment strategies in five African countries
Abstract
Background: The Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) was established in 2008 to answer strategic questions about schistosomiasis control. For programme managers, a high-priority question is: what are the most cost-effective strategies for delivering preventive chemotherapy (PCT) with praziquantel (PZQ)? This paper describes the process SCORE used to transform this question into a harmonized research protocol, the study design for answering this question, the village eligibility assessments and data resulting from the first year of the study.
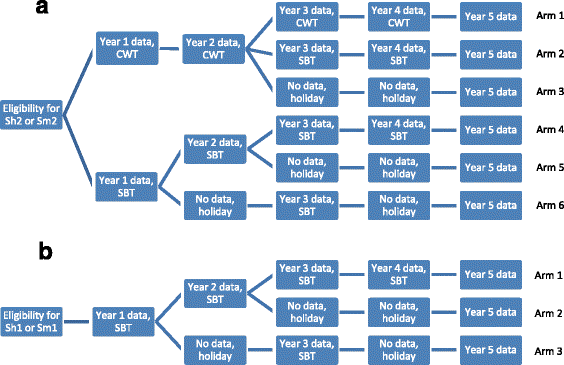
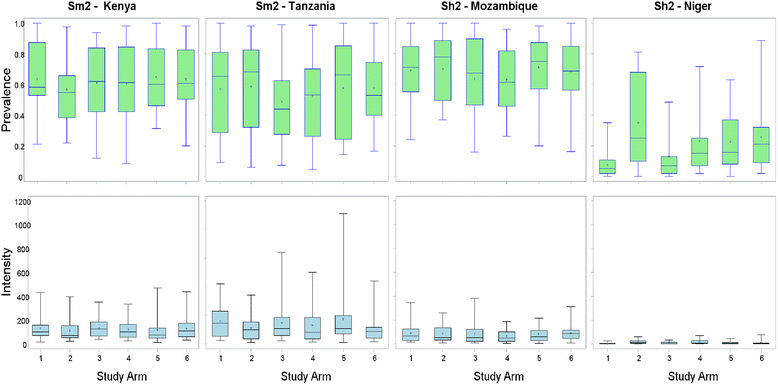
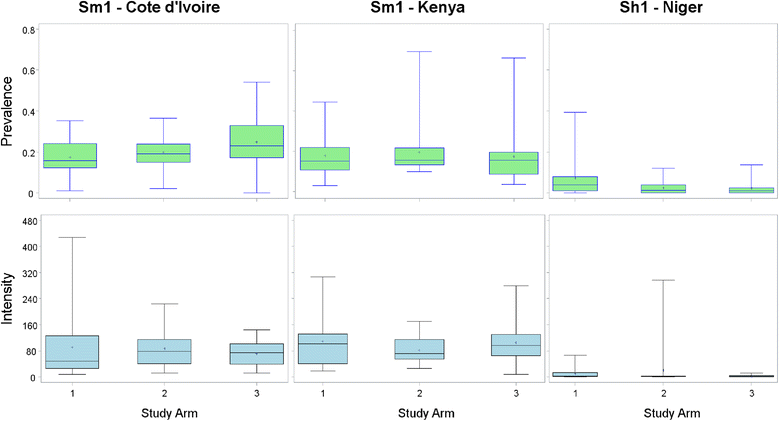
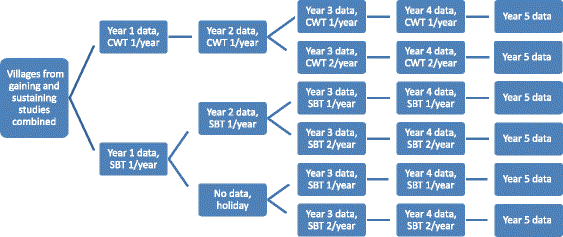
Methods: Beginning in 2009, SCORE held a series of meetings to specify empirical questions and design studies related to different schedules of PCT for schistosomiasis control in communities with high (gaining control studies) and moderate (sustaining control studies) prevalence of Schistosoma infection among school-aged children. Seven studies are currently being implemented in five African countries. During the first year, villages were screened for eligibility, and data were collected on prevalence and intensity of infection prior to randomisation and the implementation of different schemes of PZQ intervention strategies.
Results: These studies of different treatment schedules with PZQ will provide the most comprehensive data thus far on the optimal frequency and continuity of PCT for schistosomiasis infection and morbidity control.
Conclusions: We expect that the study outcomes will provide data for decision-making for country programme managers and a rich resource of information to the schistosomiasis research community.
Trial registration: The trials are registered at International Standard Randomised Controlled Trial registry (identifiers: ISRCTN99401114 , ISRCTN14849830 , ISRCTN16755535 , ISRCTN14117624 , ISRCTN95819193 and ISRCTN32045736 ).
Keywords: Control; Côte d’Ivoire; Kenya; Mozambique; Niger; Praziquantel; Preventive chemotherapy; Schistosoma haematobium; Schistosoma mansoni; Schistosomiasis; Tanzania.
Figures
References
-
- Coulibaly JT, Knopp S, N’Guessan NA, Silué KD, Fürst T, Lohourignon LK, et al. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Côte d’Ivoire. PLoS Negl Trop Dis. 2011;5(11):e1384. doi: 10.1371/journal.pntd.0001384. - DOI - PMC - PubMed
-
- Knopp S, Corstjens PL, Koukounari A, Cercamondi CI, Ame SM, Ali SM, et al. Sensitivity and specificity of a urine circulating anodic antigen test for the diagnosis of Schistosoma haematobium in low endemic settings. PLoS Negl Trop Dis. 2015;9(5):e0003752. doi: 10.1371/journal.pntd.0003752. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

