Connexins and pannexins in neuronal development and adult neurogenesis
- PMID: 27230672
- PMCID: PMC4896249
- DOI: 10.1186/s12860-016-0089-5
Connexins and pannexins in neuronal development and adult neurogenesis
Abstract
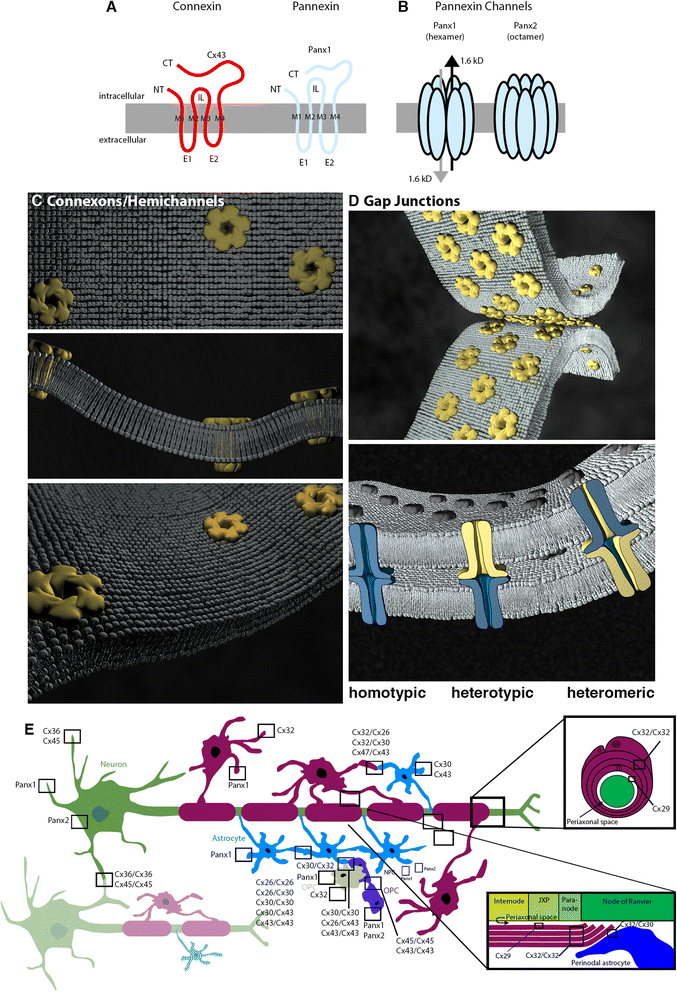
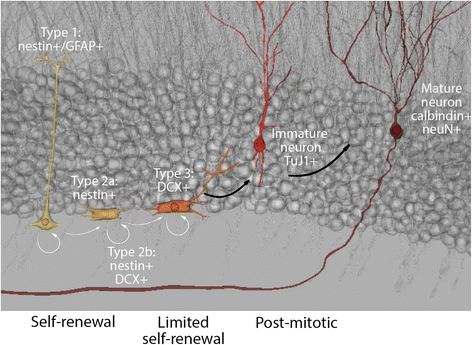
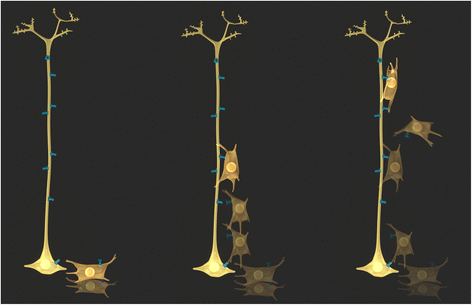
Connexins and pannexins share very similar structures and functions; they also exhibit overlapping expression in many stages of neuronal development. Here, we review evidence implicating connexin- and pannexin-mediated communication in the regulation of the birth and development of neurons, specifically Cx26, Cx30, Cx32, Cx36, Cx43, Cx45, Panx1, and Panx2. We begin by dissecting the involvement of these proteins in the generation and development of new neurons in the embryonic, postnatal, and adult brain. Next we briefly outline common mechanisms employed by both pannexins and connexins in these roles, including modulation of purinergic receptor signalling and signalling nexus functions. Throughout this review we highlight developing themes as well as important gaps in knowledge to be bridged.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

