Isocitrate Dehydrogenase Mutations Confer Dasatinib Hypersensitivity and SRC Dependence in Intrahepatic Cholangiocarcinoma
- PMID: 27231123
- PMCID: PMC5458737
- DOI: 10.1158/2159-8290.CD-15-1442
Isocitrate Dehydrogenase Mutations Confer Dasatinib Hypersensitivity and SRC Dependence in Intrahepatic Cholangiocarcinoma
Abstract
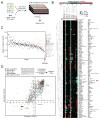
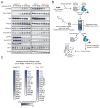
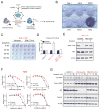
Intrahepatic cholangiocarcinoma (ICC) is an aggressive liver bile duct malignancy exhibiting frequent isocitrate dehydrogenase (IDH1/IDH2) mutations. Through a high-throughput drug screen of a large panel of cancer cell lines, including 17 biliary tract cancers, we found that IDH mutant (IDHm) ICC cells demonstrate a striking response to the multikinase inhibitor dasatinib, with the highest sensitivity among 682 solid tumor cell lines. Using unbiased proteomics to capture the activated kinome and CRISPR/Cas9-based genome editing to introduce dasatinib-resistant "gatekeeper" mutant kinases, we identified SRC as a critical dasatinib target in IDHm ICC. Importantly, dasatinib-treated IDHm xenografts exhibited pronounced apoptosis and tumor regression. Our results show that IDHm ICC cells have a unique dependency on SRC and suggest that dasatinib may have therapeutic benefit against IDHm ICC. Moreover, these proteomic and genome-editing strategies provide a systematic and broadly applicable approach to define targets of kinase inhibitors underlying drug responsiveness.
Significance: IDH mutations define a distinct subtype of ICC, a malignancy that is largely refractory to current therapies. Our work demonstrates that IDHm ICC cells are hypersensitive to dasatinib and critically dependent on SRC activity for survival and proliferation, pointing to new therapeutic strategies against these cancers. Cancer Discov; 6(7); 727-39. ©2016 AACR.This article is highlighted in the In This Issue feature, p. 681.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–14. - PubMed
-
- Hainsworth JD, Rubin MS, Spigel DR, Boccia RV, Raby S, Quinn R, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:217–23. - PubMed
-
- Varadhachary GR, Raber MN. Cancer of unknown primary site. The New England journal of medicine. 2014;371:757–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

