Modulation of Macrophage Inflammatory Nuclear Factor κB (NF-κB) Signaling by Intracellular Cryptococcus neoformans
- PMID: 27231343
- PMCID: PMC4957046
- DOI: 10.1074/jbc.M116.738187
Modulation of Macrophage Inflammatory Nuclear Factor κB (NF-κB) Signaling by Intracellular Cryptococcus neoformans
Abstract
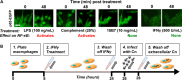
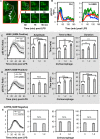
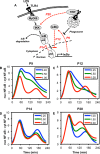
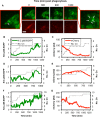
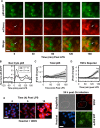
Cryptococcus neoformans (Cn) is a common facultative intracellular pathogen that can cause life-threatening fungal meningitis in immunocompromised individuals. Shortly after infection, Cn is detectable as both extra- and intracellular yeast particles, with Cn being capable of establishing long-lasting latent infections within host macrophages. Although recent studies have shown that shed capsular polysaccharides and intact extracellular Cn can compromise macrophage function through modulation of NF-κB signaling, it is currently unclear whether intracellular Cn also affects NF-κB signaling. Utilizing live cell imaging and computational modeling, we find that extra- and intracellular Cn support distinct modes of NF-κB signaling in cultured murine macrophages. Specifically, in RAW 264.7 murine macrophages treated with extracellular glucuronoxylomannan (GXM), the major Cn capsular polysaccharide, LPS-induced nuclear translocation of p65 is inhibited, whereas in cells with intracellular Cn, LPS-induced nuclear translocation of p65 is both amplified and sustained. Mathematical simulations and quantification of nascent protein expression indicate that this is a possible consequence of Cn-induced "translational interference," impeding IκBα resynthesis. We also show that long term Cn infection induces stable nuclear localization of p65 and IκBα proteins in the absence of additional pro-inflammatory stimuli. In this case, nuclear localization of p65 is not accompanied by TNFα or inducible NOS (iNOS) expression. These results demonstrate that capsular polysaccharides and intact intracellular yeast manipulate NF-κB via multiple distinct mechanisms and provide new insights into how Cn might modulate cellular signaling at different stages of an infection.
Keywords: Cryptococcus neoformans; NF-kappa B (NF-KB); computational biology; host-pathogen interaction; inflammation; intracellular pathogen; macrophage.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Lin X., and Heitman J. (2006) The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 60, 69–105 - PubMed
-
- Goldman D. L., Khine H., Abadi J., Lindenberg D. J., Pirofski L., Niang R., and Casadevall A. (2001) Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107, E66. - PubMed
-
- Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., and Chiller T. M. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

