Optically trapping tumor cells to assess differentiation and prognosis of cancers
- PMID: 27231599
- PMCID: PMC4866466
- DOI: 10.1364/BOE.7.000943
Optically trapping tumor cells to assess differentiation and prognosis of cancers
Abstract
We report an optical trapping method that may enable assessment of the differentiation status of cancerous cells by determining the minimum time required for cell-cell adhesion to occur. A single, live cell is trapped and brought into close proximity of another; the minimum contact time required for cell-cell adhesion to occur is measured using transformed cells from neural tumor cell lines: the human neuroblastoma SK-N-SH and rat C6 glioma cells. Earlier work on live adult rat hippocampal neural progenitors/stem cells had shown that a contact minimum of ~5 s was required for cells to adhere to each other. We now find the average minimum time for adhesion of cells from both tumor cell lines to substantially increase to ~20-25 s, in some cases up to 45 s. Upon in vitro differentiation of these cells with all-trans retinoic acid the average minimum time reverts to ~5-7 s. This proof-of-concept study indicates that optical trapping may be a quick, sensitive, and specific method for determining differentiation status and, thereby, the prognosis of cancer cells.
Keywords: (000.1430) Biology and medicine; (170.1530) Cell analysis.
Figures
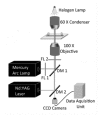
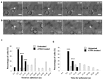
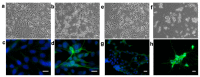
References
-
- Mathur D., “Biology-inspired AMO physics,” J. Phys. B 48(2), 022001 (2015).10.1088/0953-4075/48/2/022001 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
