In vivo bioluminescence and reflectance imaging of multiple organs in bioluminescence reporter mice by bundled-fiber-coupled microscopy
- PMID: 27231601
- PMCID: PMC4866468
- DOI: 10.1364/BOE.7.000963
In vivo bioluminescence and reflectance imaging of multiple organs in bioluminescence reporter mice by bundled-fiber-coupled microscopy
Abstract
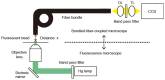
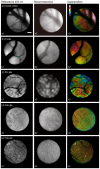
Bioluminescence imaging (BLI) is used in biomedical research to monitor biological processes within living organisms. Recently, fiber bundles with high transmittance and density have been developed to detect low light with high resolution. Therefore, we have developed a bundled-fiber-coupled microscope with a highly sensitive cooled-CCD camera that enables the BLI of organs within the mouse body. This is the first report of in vivo BLI of the brain and multiple organs in luciferase-reporter mice using bundled-fiber optics. With reflectance imaging, the structures of blood vessels and organs can be seen clearly with light illumination, and it allowed identification of the structural details of bioluminescence images. This technique can also be applied to clinical diagnostics in a low invasive manner.
Keywords: (110.2350) Fiber optics imaging; (170.2150) Endoscopic imaging; (170.3880) Medical and biological imaging.
Figures







References
-
- Deluca M., “Firefly luciferase,” Adv. Enzymol. Relat. Areas Mol. Biol. 44, 37–68 (1976). - PubMed
-
- Wood K. V., “The chemical mechanism and evolutionary development of beetle bioluminescence,” Photochem. Photobiol. 62(4), 662–673 (1995).10.1111/j.1751-1097.1995.tb08714.x - DOI
-
- Shimomura O., Bioluminescence: Chemical Principles and Methods (World Scientific, Hackensack, N.J., 2006), pp. xxvii, 470 p.
LinkOut - more resources
Full Text Sources
Other Literature Sources
