Optimization of viral resuspension methods for carbon-rich soils along a permafrost thaw gradient
- PMID: 27231649
- PMCID: PMC4878379
- DOI: 10.7717/peerj.1999
Optimization of viral resuspension methods for carbon-rich soils along a permafrost thaw gradient
Abstract
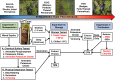
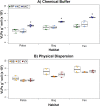
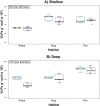
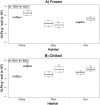
Permafrost stores approximately 50% of global soil carbon (C) in a frozen form; it is thawing rapidly under climate change, and little is known about viral communities in these soils or their roles in C cycling. In permafrost soils, microorganisms contribute significantly to C cycling, and characterizing them has recently been shown to improve prediction of ecosystem function. In other ecosystems, viruses have broad ecosystem and community impacts ranging from host cell mortality and organic matter cycling to horizontal gene transfer and reprogramming of core microbial metabolisms. Here we developed an optimized protocol to extract viruses from three types of high organic-matter peatland soils across a permafrost thaw gradient (palsa, moss-dominated bog, and sedge-dominated fen). Three separate experiments were used to evaluate the impact of chemical buffers, physical dispersion, storage conditions, and concentration and purification methods on viral yields. The most successful protocol, amended potassium citrate buffer with bead-beating or vortexing and BSA, yielded on average as much as 2-fold more virus-like particles (VLPs) g(-1) of soil than other methods tested. All method combinations yielded VLPs g(-1) of soil on the 10(8) order of magnitude across all three soil types. The different storage and concentration methods did not yield significantly more VLPs g(-1) of soil among the soil types. This research provides much-needed guidelines for resuspending viruses from soils, specifically carbon-rich soils, paving the way for incorporating viruses into soil ecology studies.
Keywords: Active layer; Humic-laden; Microbial ecology; Peatland; Permafrost; Phages; Soil viruses; Viral diversity; Viral ecology; Viral methods.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

