A Systematic Review of PCSK9 Inhibitors Alirocumab and Evolocumab
- PMID: 27231792
- PMCID: PMC10397903
- DOI: 10.18553/jmcp.2016.22.6.641
A Systematic Review of PCSK9 Inhibitors Alirocumab and Evolocumab
Abstract
Background: The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are a new class of cholesterol-lowering medications that provide significant reductions in lipids but at a large cost relative to statins. With 2 such drugs now on the market, alirocumab and evolocumab, comparing the evidence base for these drugs is necessary for informed decision making.
Objective: To compare the benefits and harms of the PCSK9 inhibitors alirocumab and evolocumab.
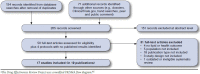
Methods: The databases Ovid MEDLINE, Cochrane Library, SCOPUS, and ClinicalTrials.gov were used to search for randomized controlled trials of alirocumab or evolocumab with any relevant comparator reporting health outcomes, lipid outcomes, or harms through September 2015, and information was requested from manufacturers. Results were reviewed according to standard review methods.
Results: The database searches revealed 17 fair- and good-quality trials; however, none had primary health outcomes or directly compared PCSK9 inhibitors. Alirocumab (75 mg to 150 mg subcutaneously every 2 weeks) resulted in significantly greater reductions in low-density lipoprotein cholesterol (LDL-C; -8% to -67%) at 12-24 weeks in patients with (a) heterozygous familial hypercholesterolemia and (b) patients at high or varied cardiovascular (CV) risk who were not at LDL-C goals with statin therapy. The highest strength evidence was for patients with high CV risk not at LDL-C goals. Alirocumab also resulted in high-density lipoprotein cholesterol (HDL-C) increases of 6%-12%. Low- and moderate-strength evidence for adjudicated CV events at 52-78 weeks for a priori analyses indicated no benefit. Low- and moderate-strength evidence also found no differences in harms except possibly slightly more injection-site reactions. Evolocumab (120 mg subcutaneously every 2 weeks to 420 mg every 4 weeks) resulted in significantly greater reductions in LDL-C (-32% to -71%) at 12-52 weeks in patients with heterozygous or homozygous familial hypercholesterolemia, patients intolerant of statins, and patients with varied CV risk not at LDL-C goal with statin therapy. The highest strength evidence was for heterozygous familial hypercholesterolemia and patients not at LDL-C goals. Moderate-strength evidence showed HDL-C increases in the range of 4.5%-6.8%. Harms were not different between groups, except possibly slightly greater overall adverse event reporting. Evidence on adjudicated CV outcomes was insufficient to draw conclusions because of sparseness of events, study limitations, and inability to assess consistency of findings.
Conclusions: Alirocumab and evolocumab have evidence of large improvements in lipid levels. The strength of the evidence is greater for alirocumab than evolocumab in patients with high CV risk who were not at LDL-C target goals, while evidence for evolocumab is stronger in patients with heterogeneous familial hypercholesterolemia and patients with varied CV risk who were not at LDL-C target goals. Evidence on adjudicated CV outcomes for a priori analyses is unable to show benefit for alirocumab and is insufficient to draw conclusions for evolocumab. Important questions remain about the comparative effects on long-term health outcomes.
Disclosures: This project was funded by The Drug Effectiveness Review Project. Project participants reviewed the manuscript but had no role in conducting the work or writing the manuscript. Any comments received from the participants during the course of the review were taken at the discretion of the authors independently. All authors had access to the data and a role in writing the manuscript. McDonagh, Peterson, and Holzhammer declare no conflict of interest or financial interest in any therapy discussed in this article. Fazio declares receiving compensation from Sanofi for a presentation on his science to a group of their advisors and has served as a consultant to MSD, BASF, NHP, Sanofi, Ionis Pharmaceuticals, and Kowa. Study concept and design were primarily contributed by McDonagh, along with Peterson and Holzhammer, with assistance from Fazio. Holzhammer took the lead in data collection, with assistance from McDonagh and Peterson. Data interpretation was performed by McDonagh, Peterson, and Fazio. The manuscript was written by McDonagh, Peterson, and Fazio, with assistance from Holzhammer, and revised by all the authors.
Conflict of interest statement
This project was funded by The Drug Effectiveness Review Project. Project participants reviewed the manuscript but had no role in conducting the work or writing the manuscript. Any comments received from the participants during the course of the review were taken at the discretion of the authors independently. All authors had access to the data and a role in writing the manuscript. McDonagh, Peterson, and Holzhammer declare no conflict of interest or financial interest in any therapy discussed in this article. Fazio declares receiving compensation from Sanofi for a presentation on his science to a group of their advisors and has served as a consultant to MSD, BASF, NHP, Sanofi, Ionis Pharmaceuticals, and Kowa.
Study concept and design were primarily contributed by McDonagh, along with Peterson and Holzhammer, with assistance from Fazio. Holzhammer took the lead in data collection, with assistance from McDonagh and Peterson. Data interpretation was performed by McDonagh, Peterson, and Fazio. The manuscript was written by McDonagh, Peterson, and Fazio, with assistance from Holzhammer, and revised by all the authors.
Similar articles
-
PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease.Cochrane Database Syst Rev. 2017 Apr 28;4(4):CD011748. doi: 10.1002/14651858.CD011748.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Oct 20;10:CD011748. doi: 10.1002/14651858.CD011748.pub3. PMID: 28453187 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations.BMJ. 2022 May 4;377:e069066. doi: 10.1136/bmj-2021-069066. BMJ. 2022. PMID: 35508320
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Incremental net benefit of lipid-lowering therapy with PCSK9 inhibitors: a systematic review and meta-analysis of cost-utility studies.Eur J Clin Pharmacol. 2022 Mar;78(3):351-363. doi: 10.1007/s00228-021-03242-6. Epub 2021 Oct 27. Eur J Clin Pharmacol. 2022. PMID: 34708270
-
Successful use of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors in Hypertriglyceridemia-induced Acute Pancreatitis: A Case Report.Curr Cardiol Rev. 2025;21(3):e1573403X343784. doi: 10.2174/011573403X343784241115055037. Curr Cardiol Rev. 2025. PMID: 39773061
-
Randomised, phase 1, dose-finding study of MEDI4166, a PCSK9 antibody and GLP-1 analogue fusion molecule, in overweight or obese patients with type 2 diabetes mellitus.Diabetologia. 2019 Mar;62(3):373-386. doi: 10.1007/s00125-018-4789-6. Epub 2018 Dec 28. Diabetologia. 2019. PMID: 30593607 Clinical Trial.
-
Immune-based Therapies-What the Emergency Physician Needs to Know.Emerg Med Clin North Am. 2022 Feb;40(1):135-148. doi: 10.1016/j.emc.2021.08.011. Epub 2021 Oct 29. Emerg Med Clin North Am. 2022. PMID: 34782084 Free PMC article. Review.
-
Management of Statin Intolerant Patients in the Era of Novel Lipid Lowering Therapies: A Critical Approach in Clinical Practice.J Clin Med. 2023 Mar 22;12(6):2444. doi: 10.3390/jcm12062444. J Clin Med. 2023. PMID: 36983444 Free PMC article. Review.
References
-
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-97. - PubMed
-
- Evolocumab (Repatha)—a second PCSK9 inhibitor to lower LDL-cholesterol. JAMA. 2015;314(21):2298-99. - PubMed
-
- Carroll J. UPDATED: Amgen gets a big win with FDA OK for PCSK9 cholesterol drug Repatha. FierceBiotech. August 27, 2015. Available at: http://www.fiercebiotech.com/story/amgen-gets-big-win-fda-ok-pcsk9-drug-.... Accessed April 12, 2016.
-
- Pollack A. New drug sharply lowers cholesterol, but it's costly. The New York Times. July 24, 2015. Available at: http://www.nytimes.com/2015/07/25/business/us-approves-drug-that-can-sha.... Accessed April 12, 2016.
References for Appendices A and B
-
- Cannon CP, Cariou B, Blom D, et al. . Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholes-terolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36(19):1186-94. - PMC - PubMed
-
- Kereiakes DJ, Robinson JG, Cannon CP, et al. . Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169(6):906-15.e913. - PubMed
-
- McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand A-C, Stein EA.. Safety and efficacy of a monoclonal antibody to proprotein convertase sub-tilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59(25):2344-53. - PubMed
-
- Robinson JG, Farnier M, Krempf M, et al. . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489-99. - PubMed
-
- Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA.. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367(20):1891-900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous