Medication Reconciliation in Oncological Patients: A Randomized Clinical Trial
- PMID: 27231800
- PMCID: PMC10398067
- DOI: 10.18553/jmcp.2016.15248
Medication Reconciliation in Oncological Patients: A Randomized Clinical Trial
Abstract
Background: Medication reconciliation is considered to be an important strategy for increasing the safety of medication use. However, few studies have been carried out showing the effect of a medication reconciliation program on the incidence of reconciliation errors (REs) in oncological patients treated in the outpatient setting.
Objective: To measure the effect of a medication reconciliation program on the incidence of reconciliation error that reached the patient (RERP) in cancer patients receiving chemotherapy as outpatients.
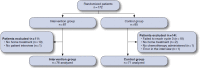
Methods: A randomized, prospective, controlled study was carried out to identify the proportion of patients with at least 1 RERP. Medication reconciliation (intervention group) was compared with standard practice (control group) in patients starting new chemotherapy and who were receiving at least 1 home medication before the start of chemotherapy. A prespecified analysis of factors capable of influencing the occurrence of RE in oncological patients was also carried out.
Results: A total of 147 patients were included (76 in the intervention group and 71 controls) in this study. There were 3 (4%) patients with RERP (primary endpoint) in the intervention group and 21 (30%) patients in the control group (relative risk [RR] = 0.13, 95% CI = 0.04-0.43; P = 0.0009). The prespecified analysis of the effects of the Eastern Cooperative Oncology Group performance status (ECOG), Charlson Comorbidity Index score, and degree of poly-medication upon the number of patients with RE showed the Charlson Comorbidity Index to be unrelated to RE occurrence. However, the risk of RE was greater in patients with ECOG ≥ 2 (RR = 2.18, 95% CI = 1.4-3.4; P = 0.018) and among patients with major poly-medication (RR = 2.49, 95% CI = 1.52-4.09; P <0.001).
Conclusions: Medication reconciliation results in a marked decrease in RERP in cancer patients. The factors that may influence RE occurrence in oncological patients have not been fully established, although parameters such as the degree of poly-medication and performance status may play a role.
Disclosures: No outside funding supported this study. The authors declare that they have no affiliations with or financial interests in any company, product, or service described in the manuscript. Study concept and design were contributed by Sierra-Sánchez, Martínez-Bautista, Baena-Cañada, and González-Carrascosa Vega. Martínez-Bautista, García-Martín, Suárez-Carrascosa, and González-Carrascosa Vega collected the data, which was interpreted by Sierra-Sánchez, Martínez-Bautista, Baena-Cañada, and González-Carrascosa Vega. The manuscript was written by Sierra-Sánchez and González-Carrascosa Vega and revised by Sierra-Sánchez, Martínez-Bautista, Baena-Cañada, and González-Carrascosa Vega.
Conflict of interest statement
No outside funding supported this study. The authors declare that they have no affiliations with or financial interests in any company, product, or service described in the manuscript.
Study concept and design were contributed by Sierra-Sánchez, Martínez-Bautista, Baena-Cañada, and González-Carrascosa Vega. Martínez-Bautista, García-Martín, Suárez-Carrascosa, and González-Carrascosa Vega collected the data, which was interpreted by Sierra-Sánchez, Martínez-Bautista, Baena-Cañada, and González-Carrascosa Vega. The manuscript was written by Sierra-Sánchez and González-Carrascosa Vega and revised by Sierra-Sánchez, Martínez-Bautista, Baena-Cañada, and González-Carrascosa Vega.
Figures
References
-
- Joint Commission Accreditation Healthcare Organizations.. Comprehensive Accreditation Manual for Hospitals (CAMH): The Official Handbook. Oakbrook Terrace, IL: Joint Commission Resources, 2006.
-
- Institute for Healthcare Improvement.. How-to guide: prevent adverse drug events (medication reconciliation). Revised March 2016. Available at: http://www.ihi.org/resources/Pages/Tools/HowtoGuidePreventAdverseDrugEve.... Accessed April 3, 2016.
-
- Gleason KM, Brake H, Agramonte V, Perfetti C.. Medications at transitions and clinical handoffs (MATCH) toolkit for medication reconciliation. (Prepared by the Island Peer Review Organization, Inc., under Contract No. HHSA2902009000 13C). AHRQ Publication No. 11(12)-0059. Rockville, MD: Agency for Healthcare Research and Quality. Revised August 2012. Available at: http://www.ahrq.gov/sites/default/files/publications/files/match.pdf. Accessed March 18, 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical