Adapting the Stress Response: Viral Subversion of the mTOR Signaling Pathway
- PMID: 27231932
- PMCID: PMC4926172
- DOI: 10.3390/v8060152
Adapting the Stress Response: Viral Subversion of the mTOR Signaling Pathway
Abstract
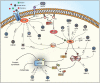
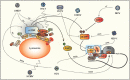
The mammalian target of rapamycin (mTOR) is a central regulator of gene expression, translation and various metabolic processes. Multiple extracellular (growth factors) and intracellular (energy status) molecular signals as well as a variety of stressors are integrated into the mTOR pathway. Viral infection is a significant stress that can activate, reduce or even suppress the mTOR signaling pathway. Consequently, viruses have evolved a plethora of different mechanisms to attack and co-opt the mTOR pathway in order to make the host cell a hospitable environment for replication. A more comprehensive knowledge of different viral interactions may provide fruitful targets for new antiviral drugs.
Keywords: 4EBP1; Akt; PI3K; autophagy; mTOR; virus.
Figures
References
-
- Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

