Cuminaldehyde from Cinnamomum verum Induces Cell Death through Targeting Topoisomerase 1 and 2 in Human Colorectal Adenocarcinoma COLO 205 Cells
- PMID: 27231935
- PMCID: PMC4924159
- DOI: 10.3390/nu8060318
Cuminaldehyde from Cinnamomum verum Induces Cell Death through Targeting Topoisomerase 1 and 2 in Human Colorectal Adenocarcinoma COLO 205 Cells
Abstract
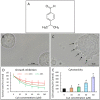
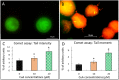
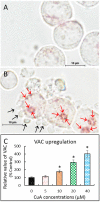
Cinnamomum verum, also called true cinnamon tree, is employed to make the seasoning cinnamon. Furthermore, the plant has been used as a traditional Chinese herbal medication. We explored the anticancer effect of cuminaldehyde, an ingredient of the cortex of the plant, as well as the molecular biomarkers associated with carcinogenesis in human colorectal adenocarcinoma COLO 205 cells. The results show that cuminaldehyde suppressed growth and induced apoptosis, as proved by depletion of the mitochondrial membrane potential, activation of both caspase-3 and -9, and morphological features of apoptosis. Moreover, cuminaldehyde also led to lysosomal vacuolation with an upregulated volume of acidic compartment and cytotoxicity, together with inhibitions of both topoisomerase I and II activities. Additional study shows that the anticancer activity of cuminaldehyde was observed in the model of nude mice. Our results suggest that the anticancer activity of cuminaldehyde in vitro involved the suppression of cell proliferative markers, topoisomerase I as well as II, together with increase of pro-apoptotic molecules, associated with upregulated lysosomal vacuolation. On the other hand, in vivo, cuminaldehyde diminished the tumor burden that would have a significant clinical impact. Furthermore, similar effects were observed in other tested cell lines. In short, our data suggest that cuminaldehyde could be a drug for chemopreventive or anticancer therapy.
Keywords: antiproliferative; cuminaldehyde; lysosomal vacuolation; topoisomerase I; topoisomerase II; xenograft.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

