AVN-101: A Multi-Target Drug Candidate for the Treatment of CNS Disorders
- PMID: 27232215
- PMCID: PMC4969713
- DOI: 10.3233/JAD-151146
AVN-101: A Multi-Target Drug Candidate for the Treatment of CNS Disorders
Abstract
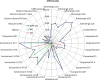
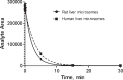
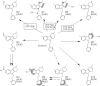
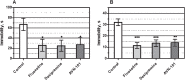
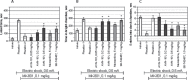
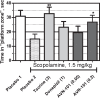
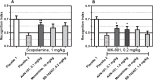
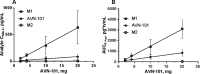
Lack of efficacy of many new highly selective and specific drug candidates in treating diseases with poorly understood or complex etiology, as are many of central nervous system (CNS) diseases, encouraged an idea of developing multi-modal (multi-targeted) drugs. In this manuscript, we describe molecular pharmacology, in vitro ADME, pharmacokinetics in animals and humans (part of the Phase I clinical studies), bio-distribution, bioavailability, in vivo efficacy, and safety profile of the multimodal drug candidate, AVN-101. We have carried out development of a next generation drug candidate with a multi-targeted mechanism of action, to treat CNS disorders. AVN-101 is a very potent 5-HT7 receptor antagonist (Ki = 153 pM), with slightly lesser potency toward 5-HT6, 5-HT2A, and 5HT-2C receptors (Ki = 1.2-2.0 nM). AVN-101 also exhibits a rather high affinity toward histamine H1 (Ki = 0.58 nM) and adrenergic α2A, α2B, and α2C (Ki = 0.41-3.6 nM) receptors. AVN-101 shows a good oral bioavailability and facilitated brain-blood barrier permeability, low toxicity, and reasonable efficacy in animal models of CNS diseases. The Phase I clinical study indicates the AVN-101 to be well tolerated when taken orally at doses of up to 20 mg daily. It does not dramatically influence plasma and urine biochemistry, nor does it prolong QT ECG interval, thus indicating low safety concerns. The primary therapeutic area for AVN-101 to be tested in clinical trials would be Alzheimer's disease. However, due to its anxiolytic and anti-depressive activities, there is a strong rational for it to also be studied in such diseases as general anxiety disorders, depression, schizophrenia, and multiple sclerosis.
Keywords: 5-HT7 receptor antagonists; Adrenergic alpha-2 antagonists; Alzheimer’s disease; Parkinson’s disease; anxiety; central nervous system agents; histamine H1 receptor antagonists; memory; serotonin receptor antagonists.
Figures







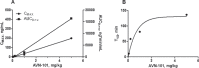













Similar articles
-
AVN-492, A Novel Highly Selective 5-HT6R Antagonist: Preclinical Evaluation.J Alzheimers Dis. 2017;58(4):1043-1063. doi: 10.3233/JAD-161262. J Alzheimers Dis. 2017. PMID: 28550249
-
AVN-322 is a Safe Orally Bio-Available Potent and Highly Selective Antagonist of 5-HT6R with Demonstrated Ability to Improve Impaired Memory in Animal Models.Curr Alzheimer Res. 2017;14(3):268-294. doi: 10.2174/1567205013666161108105005. Curr Alzheimer Res. 2017. PMID: 27829340
-
From anti-allergic to anti-Alzheimer's: Molecular pharmacology of Dimebon.Curr Alzheimer Res. 2010 Mar;7(2):97-112. doi: 10.2174/156720510790691100. Curr Alzheimer Res. 2010. PMID: 19939222
-
Serotonergic 5-HT6 Receptor Antagonists: Heterocyclic Chemistry and Potential Therapeutic Significance.Curr Top Med Chem. 2015;15(17):1643-62. doi: 10.2174/1568026615666150427110420. Curr Top Med Chem. 2015. PMID: 25915615 Review.
-
The serotonin 5-HT6 receptor: a viable drug target for treating cognitive deficits in Alzheimer's disease.Expert Rev Neurother. 2009 Jul;9(7):1073-85. doi: 10.1586/ern.09.51. Expert Rev Neurother. 2009. PMID: 19589055 Review.
Cited by
-
Strategies for Treatment of Disease-Associated Dementia Beyond Alzheimer's Disease: An Update.Curr Neuropharmacol. 2023;21(2):309-339. doi: 10.2174/1570159X20666220411083922. Curr Neuropharmacol. 2023. PMID: 35410602 Free PMC article. Review.
-
Multi-Target Approach for Drug Discovery against Schizophrenia.Int J Mol Sci. 2018 Oct 10;19(10):3105. doi: 10.3390/ijms19103105. Int J Mol Sci. 2018. PMID: 30309037 Free PMC article. Review.
-
The Histamine and Multiple Sclerosis Alliance: Pleiotropic Actions and Functional Validation.Curr Top Behav Neurosci. 2022;59:217-239. doi: 10.1007/7854_2021_240. Curr Top Behav Neurosci. 2022. PMID: 34432258
-
In Silico Screening of Natural Compounds for Candidates 5HT6 Receptor Antagonists against Alzheimer's Disease.Molecules. 2022 Apr 19;27(9):2626. doi: 10.3390/molecules27092626. Molecules. 2022. PMID: 35565976 Free PMC article.
-
5HT6 Antagonists in the Treatment of Alzheimer's Dementia: Current Progress.Neurol Ther. 2018 Jun;7(1):51-58. doi: 10.1007/s40120-018-0095-y. Epub 2018 May 2. Neurol Ther. 2018. PMID: 29728891 Free PMC article. Review.
References
-
- Lipinski CA (2012) Phenotypic and in vivo screening: Lead discovery and drug repurposing In, Designing Multi-Target Drugs, Morphy JR, Harris CJ, eds. Royal Society of Chemistry, pp, 86–93.
-
- Hornberg JJ (2012) Simple drugs do not cure complex diseases: The need for multi-targeted drugs. In Designing Multi-Target Drugs, Morphy JR, Harris CJ, eds. Royal Society of Chemistry, pp, 1–13.
-
- (2015) 2015 Alzheimer’s disease facts and figures. Alzheimers Dement 11, 332–384. - PubMed
-
- Schizophrenia Facts and Statistics, http://www.schizophrenia.com/szfacts.htm.
-
- Anxiety and Depression Association of America. Facts & Statistics, http://www.adaa.org/about-adaa/press-room/facts-statistics, Last updated September, 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

