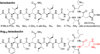Elucidation of the Teixobactin Pharmacophore
- PMID: 27232661
- PMCID: PMC5283680
- DOI: 10.1021/acschembio.6b00295
Elucidation of the Teixobactin Pharmacophore
Abstract
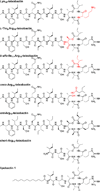
This paper elucidates the teixobactin pharmacophore by comparing the arginine analogue of teixobactin Arg10-teixobactin to seven homologues with varying structure and stereochemistry. The roles of the guanidinium group at position 10, the stereochemistry of the macrolactone ring, and the "tail" comprising residues 1-5 are investigated. The guanidinium group is not necessary for activity; Lys10-teixobactin is more active than Arg10-teixobactin against Gram-positive bacteria in minimum inhibitory concentration (MIC) assays. The relative stereochemistry of the macrolactone ring is important. Diastereomer l-Thr8,Arg10-teixobactin is inactive, and diastereomer d-allo-Ile11,Arg10-teixobactin is less active. The macrolactone ring is critical; seco-Arg10-teixobactin is inactive. The absolute stereochemistry is not important; the enantiomer ent-Arg10-teixobactin is comparable in activity. The hydrophobic N-terminal tail is important. Truncation of residues 1-5 results in loss of activity, and replacement of residues 1-5 with a dodecanoyl group partially restores activity. These findings pave the way for developing simpler homologues of teixobactin with enhanced pharmacological properties.
Figures
References
-
- Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. - PMC - PubMed
-
- Editorial. Listen up, (2015) Nature. 517:121. - PubMed
-
- Ledford H. Promising antibiotic discovered in microbial ‘dark matter’. [accessed January 7, 2015];Nature News. 2015 Jan 7; [Online], http://www.nature.com/news/promising-antibiotic-discovered-in-microbial-....
-
- Wright G. Antibiotics: An irresistible newcomer. Nature. 2015;517:442–444. - PubMed
-
- Kahrstrom CT. Antibacterial drugs: a new drug for resistant bugs. Nature Rev. Drug Discov. 2015;14:94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous