Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma
- PMID: 27232884
- PMCID: PMC4883746
- DOI: 10.1371/journal.pone.0156369
Multi-Center Randomized Phase II Study Comparing Cediranib plus Gefitinib with Cediranib plus Placebo in Subjects with Recurrent/Progressive Glioblastoma
Abstract
Background: Cediranib, an oral pan-vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor, failed to show benefit over lomustine in relapsed glioblastoma. One resistance mechanism for cediranib is up-regulation of epidermal growth factor receptor (EGFR). This study aimed to determine if dual therapy with cediranib and the oral EGFR inhibitor gefitinib improved outcome in recurrent glioblastoma.
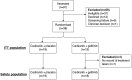
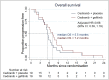
Methods and findings: This was a multi-center randomized, two-armed, double-blinded phase II study comparing cediranib plus gefitinib versus cediranib plus placebo in subjects with first relapse/first progression of glioblastoma following surgery and chemoradiotherapy. The primary outcome measure was progression free survival (PFS). Secondary outcome measures included overall survival (OS) and radiologic response rate. Recruitment was terminated early following suspension of the cediranib program. 38 subjects (112 planned) were enrolled with 19 subjects in each treatment arm. Median PFS with cediranib plus gefitinib was 3.6 months compared to 2.8 months for cediranib plus placebo (HR; 0.72, 90% CI; 0.41 to 1.26). Median OS was 7.2 months with cediranib plus gefitinib and 5.5 months with cediranib plus placebo (HR; 0.68, 90% CI; 0.39 to 1.19). Eight subjects (42%) had a partial response in the cediranib plus gefitinib arm versus five patients (26%) in the cediranib plus placebo arm.
Conclusions: Cediranib and gefitinib in combination is tolerated in patients with glioblastoma. Incomplete recruitment led to the study being underpowered. However, a trend towards improved survival and response rates with the addition of gefitinib to cediranib was observed. Further studies of the combination incorporating EGFR and VEGF inhibition are warranted.
Trial registration: ClinicalTrials.gov NCT01310855.
Conflict of interest statement
Figures
References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10(5):459–66. 10.1016/S1470-2045(09)70025-7 . - DOI - PubMed
-
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 Suppl 4:S9–15. . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous