Cryopreservation of Human Mucosal Leukocytes
- PMID: 27232996
- PMCID: PMC4883784
- DOI: 10.1371/journal.pone.0156293
Cryopreservation of Human Mucosal Leukocytes
Abstract
Background: Understanding how leukocytes in the cervicovaginal and colorectal mucosae respond to pathogens, and how medical interventions affect these responses, is important for developing better tools to prevent HIV and other sexually transmitted infections. An effective cryopreservation protocol for these cells following their isolation will make studying them more feasible.
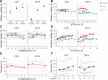
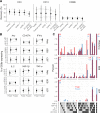
Methods and findings: To find an optimal cryopreservation protocol for mucosal mononuclear leukocytes, we compared cryopreservation media and procedures using human vaginal leukocytes and confirmed our results with endocervical and colorectal leukocytes. Specifically, we measured the recovery of viable vaginal T cells and macrophages after cryopreservation with different cryopreservation media and handling procedures. We found several cryopreservation media that led to recoveries above 75%. Limiting the number and volume of washes increased the fraction of cells recovered by 10-15%, possibly due to the small cell numbers in mucosal samples. We confirmed that our cryopreservation protocol also works well for both endocervical and colorectal leukocytes. Cryopreserved leukocytes had slightly increased cytokine responses to antigenic stimulation relative to the same cells tested fresh. Additionally, we tested whether it is better to cryopreserve endocervical cells on the cytobrush or in suspension.
Conclusions: Leukocytes from cervicovaginal and colorectal tissues can be cryopreserved with good recovery of functional, viable cells using several different cryopreservation media. The number and volume of washes has an experimentally meaningful effect on the percentage of cells recovered. We provide a detailed, step-by-step protocol with best practices for cryopreservation of mucosal leukocytes.
Conflict of interest statement
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

