Fatty acylation of proteins: The long and the short of it
- PMID: 27233110
- PMCID: PMC4975971
- DOI: 10.1016/j.plipres.2016.05.002
Fatty acylation of proteins: The long and the short of it
Abstract
Long, short and medium chain fatty acids are covalently attached to hundreds of proteins. Each fatty acid confers distinct biochemical properties, enabling fatty acylation to regulate intracellular trafficking, subcellular localization, protein-protein and protein-lipid interactions. Myristate and palmitate represent the most common fatty acid modifying groups. New insights into how fatty acylation reactions are catalyzed, and how fatty acylation regulates protein structure and function continue to emerge. Myristate is typically linked to an N-terminal glycine, but recent studies reveal that lysines can also be myristoylated. Enzymes that remove N-terminal myristoyl-glycine or myristate from lysines have now been identified. DHHC proteins catalyze S-palmitoylation, but the mechanisms that regulate substrate recognition by individual DHHC family members remain to be determined. New studies continue to reveal thioesterases that remove palmitate from S-acylated proteins. Another area of rapid expansion is fatty acylation of the secreted proteins hedgehog, Wnt and Ghrelin, by Hhat, Porcupine and GOAT, respectively. Understanding how these membrane bound O-acyl transferases recognize their protein and fatty acyl CoA substrates is an active area of investigation, and is punctuated by the finding that these enzymes are potential drug targets in human diseases.
Keywords: Depalmitoylation; Lipid rafts; MBOAT family; Myristoyl switch; Myristoylation; Palmitoylation.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
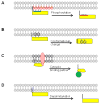
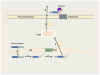
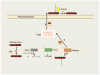
References
-
- Duronio RJ, Towler DA, Heuckeroth RO, Gordon JI. Disruption of the yeast N-myristoyl transferase gene causes recessive lethality. Science. 1989;243(4892):796–800. - PubMed
-
- Giang DK, Cravatt BF. A second mammalian N-myristoyltransferase. J Biol Chem. 1998;273(12):6595–8. - PubMed
-
- Yang SH, Shrivastav A, Kosinski C, Sharma RK, Chen MH, Berthiaume LG, Peters LL, Chuang PT, Young SG, Bergo MO. N-myristoyltransferase 1 is essential in early mouse development. J Biol Chem. 2005;280(19):18990–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

