Safety and efficacy of vernakalant for the conversion of atrial fibrillation to sinus rhythm; a phase 3b randomized controlled trial
- PMID: 27233239
- PMCID: PMC4884402
- DOI: 10.1186/s12872-016-0289-0
Safety and efficacy of vernakalant for the conversion of atrial fibrillation to sinus rhythm; a phase 3b randomized controlled trial
Abstract
Background: Atrial fibrillation (AF) is a common cardiac arrhythmia that is associated with significant health risks. One strategy to mitigate the risks associated with long-term AF is to convert AF to sinus rhythm (SR). This study assessed the efficacy and safety of vernakalant hydrochloride for the pharmacological conversion of AF to SR.
Methods: Patients with recent-onset (duration >3 h- ≤ 7 days) symptomatic AF and no evidence or history of congestive heart failure were randomized in a 2:1 ratio to receive vernakalant or placebo. Patients received an infusion of vernakalant (3 mg/kg) or placebo over 10 min, followed by a second infusion of vernakalant (2 mg/kg) or placebo 15 min later if AF had not been terminated. The primary efficacy endpoint was conversion of AF to SR for at least 1 min within 90 min of the start of drug infusion. The primary safety endpoint was a composite of: occurrence of clinically significant hypotension, clinically significant ventricular arrhythmia (including torsades de pointes, ventricular tachycardia or ventricular fibrillation) or death within 2 h of starting the drug infusion.
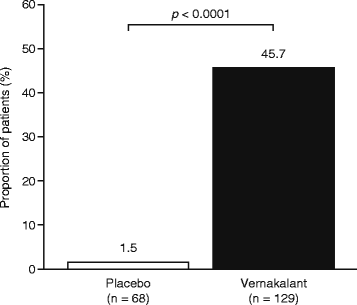
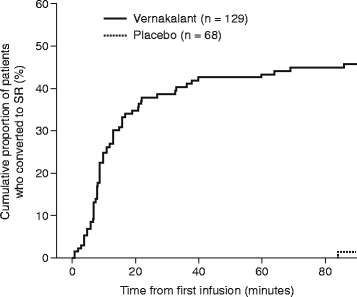
Results: A total of 217 patients were randomized to receive vernakalant (n = 145) or placebo (n = 72). Of the 129 individuals who received vernakalant, 59 (45.7 %) converted to SR compared with one of the 68 patients (1.5 %) who received placebo (p < 0.0001). Conversion to SR was significantly faster with vernakalant than with placebo (p < 0.0001), and a greater proportion of patients who received vernakalant than those who received placebo reported no AF-related symptoms at 90 min (p = 0.0264). The primary composite safety endpoint was observed in one patient receiving vernakalant and in no patients receiving placebo. In the vernakalant arm, dysgeusia, paraesthesia and sneezing were the most common treatment-emergent adverse events, and three serious adverse events occurred that were considered to be related to study drug.
Conclusions: Vernakalant resulted in rapid cardioversion of recent-onset AF in almost half of the study population and was generally well tolerated. The safety outcomes affirmed the need for careful selection and management of haemodynamically stable candidates for cardioversion.
Trial registration: NCT00989001 .
Keywords: Antiarrhythmic; Atrial fibrillation; Cardioversion; Vernakalant.
Figures
References
-
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

